Boulton-Katritzky rearrangement
The Boulton-Katritzky rearrangement was first published by its namesake A. John Boulton and Alan Katritzky in 1962. The reaction is a cyclic rearrangement of heterocycles in an acidic or basic medium or under the action of light.
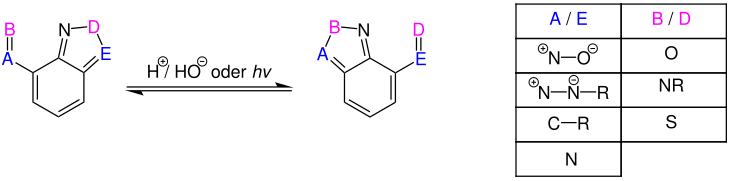
Overview reaction
The overview reaction is described in literature:
Reaction mechanism
The reaction mechanism is a [1,9] sigmatropic rearrangement, a special form of the pericyclic reaction . The mechanistic details are described in the literature:
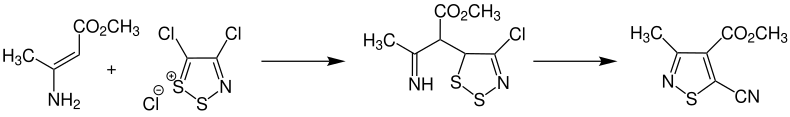
Practical use
The Boulton – Katritzky rearrangement is a key step in the synthesis of complex substituted heterocycles. For example, methyl 5-cyano-methylisothiazole-4-carboxylate is prepared from methyl 3-aminocrotonate and 4,5-dichloro-1,2,3-dithiazolium chloride.
Individual evidence
- ^ A. John Boulton, Alan Katritzky: A New Heterocyclic Rearrangement . In: Proceedings of the Chemical Society 1962, p. 257, doi: 10.1039 / PS9620000237 .
- ↑ Barbara Cosimelli, Vincenzo Frenna, Susanna Guernelli, Camilla Zaira Lanza, Gabriella Macaluso, Giovanni Petrillo, Domenico Spinelli: The First Kinetic Evidence for Acid Catalysis in a Monocyclic Rearrangement of Heterocycles: Conversion of the Z-Phenylhydrazone of 5-Amino-3- benzoyl-1,2,4-oxadiazole into N, 5-diphenyl-2H-1,2,3-triazol-4-ylurea. In: The Journal of Organic Chemistry . 67, 2002, pp. 8010-8018, doi: 10.1021 / jo026039z .
- ^ Frank Eckert, Guntram Rauhut: A Computational Study on the Reaction Mechanism of the Boulton − Katritzky Rearrangement. In: J. Am. Chem. Soc. 120 (51), 1998, pp. 13478-13484, doi: 10.1021 / ja981720x .
- ↑ KP Parry, CW Rees: A thermally degenerate [1.9] sigmatropic shift. In: J. Chem. Soc. D . , 1971, p. 833, doi: 10.1039 / C29710000833 .
- ↑ a b Zerong Wang: Comprehensive organic name reactions and reagents Volume 1 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28662-3 , pp. 482-483 .