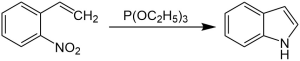Cadogan-Sundberg indole synthesis
The Cadogan-Sundberg indole synthesis is a name reaction in organic chemistry . The reaction was discovered by John Ivan George Cadogan (* 1930) and extended by Richard Jay Sundberg.
Overview reaction
In the Cadogan-Sundberg indole synthesis, an aromatic nitro compound reacts with triethyl phosphite in a cyclization reaction to form indole .
Reaction mechanism
In the proposed reaction mechanism, o -nitrostyrene ( 1 ) first reacts with triethyl phosphite, the phosphorus atom binding to the nitrogen atom of the nitro group. By splitting off triethyl phosphate, N- hydroxylindole ( 2 ) is formed, which reacts again with triethyl phosphite. Triethyl phosphate is split off and indole ( 3 ) is formed.
modification
The cyclization reaction of o -nitrostyrene has been improved by using a metal catalyst based on nickel or selenium .
application
The Cadogan-Sundberg indole synthesis can also be used for the synthesis of indole derivatives.
See also
Individual evidence
- ↑ a b c d Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, Inc., Hoboken, NJ, USA 2010, ISBN 978-0-470-63885-9 , doi : 10.1002 / 9780470638859 .
- ↑ a b c R.K. Mackie, JIG Cadogan: Tervalent phosphorus compounds in organic synthesis . In: Chemical Society Reviews . tape 3 , no. 1 , January 1, 1974, p. 87-137 , doi : 10.1039 / CS9740300087 .
- ^ A b c R. J. Sundberg: Deoxygenation of Nitro Groups by Trivalent Phosphorus. Indoles from o-nitrostyrenes . In: The Journal of Organic Chemistry . tape 30 , no. November 11 , 1965, pp. 3604-3610 , doi : 10.1021 / jo01022a006 .
- ↑ Richard J. Sundberg, Toshio Yamazaki: Rearrangements and ring expansions during the deoxygenation of .beta.,. Beta.-disubstituted o-nitrostyrenes . In: The Journal of Organic Chemistry . tape 32 , no. 2 , February 1967, p. 290-294 , doi : 10.1021 / jo01288a009 .
- ↑ Shubhada W Dantale, Björn CG Söderberg: A novel palladium-catalyzed synthesis of β-carbolines: application in total synthesis of naturally occurring alkaloids . In: Tetrahedron . tape 59 , no. 29 , 2003, p. 5507-5514 , doi : 10.1016 / S0040-4020 (03) 00824-X .
- ↑ Yutaka Nishiyama, Ryo Maema, Kengou Ohno, Masaharu Hirose, Noboru Sonoda: Synthesis of indoles: selenium-catalyzed reductive N-heterocyclization of 2-nitrostyrenes with carbon monoxide . In: Tetrahedron Letters . tape 40 , no. 31 , 1999, p. 5717-5720 , doi : 10.1016 / S0040-4039 (99) 01080-1 .
- ↑ L. Lee Melhado, Nelson J. Leonard: An efficient synthesis of azidoindoles and azidotryptophans . In: The Journal of Organic Chemistry . tape 48 , no. December 25 , 1983, pp. 5130-5133 , doi : 10.1021 / jo00173a071 .