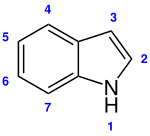
Indole
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
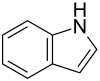
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Indole | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 7 N | |||||||||||||||||||||
| Brief description |
colorless leaflets that are purely flower-like and contaminated with an unpleasant odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 117.15 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.22 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
52 ° C |
|||||||||||||||||||||
| boiling point |
254 ° C |
|||||||||||||||||||||
| Vapor pressure |
1.6 Pa (25 ° C) |
|||||||||||||||||||||
| solubility |
heavy in water (3.56 g l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Indole is a chemical substance from the group of aromatic , heterocyclic amines and occurs as a structural fragment in many natural products .
history
Indole chemistry began with studying the widely used dye indigo . Indigo can be converted to isatin and further to oxindole . In 1866, Adolf von Baeyer reduced oxindoles to indole with the help of zinc dust . In 1869 he proposed a structural formula for indole.
Certain indole derivatives were important dyes until the end of the 19th century. Interest in indole increased in the 1930s when it became known that indole is a basic component of many important natural substances such as alkaloids (for example strychnine and auxin ) and the amino acid tryptophan and the neurotransmitters derived from it ( serotonin , melatonin ). It remains an active area of research to this day.
Occurrence
Indole is contained in jasmine blossom oil and gold lacquer oil , but also in the blossoms of false acacia and arum . In low concentrations, indole gives the typical flower scent and is therefore also added to perfumes . In higher concentrations, indole, as a breakdown product of the amino acid tryptophan , together with skatole (3-methylindole), is the cause of the typical stench of faeces . It is also in the boiling at 240 to 260 ° C coal tar - fraction contained.
Indole is part of the proteinogenic amino acid tryptophan .
presentation
Indole or indole derivatives can be represented in different ways. Among other things through:
- Japp-Klingemann reaction
- Bischler-Möhlau indole synthesis
- Larock indole synthesis
- Madelung indole synthesis
- Leimgruber batcho indole synthesis
- Nenitzescu indole synthesis
- Gassman indole synthesis
- Reissert indole synthesis
- Fürstner indole synthesis
use
Indole is the basic building block for dyes , hormones and alkaloids . The best known dye derived from indole is indigo , but the ancient dye purple is such a derivative . The indole building block is u. a. in the hormone melatonin ( N -acetyl-5-methoxytryptamine), in the neurotransmitter serotonin (5-hydroxytryptamine) and in the auxin indole-3-acetic acid . Among the alkaloids that contain indole are the strychnos alkaloids (e.g. strychnine and brucine ), the ergot alkaloids ( ergotamine and its synthetic derivative LSD ), and the mushroom ingredient psilocybin . It is also used to make artificial jasmine and neroli oils .
properties
Indole is weakly basic, but hardly forms salts with acids; instead, it readily reacts to form resinous polymers . The NH proton is abstracted by alkali metals . The pyrrole ring reacts preferentially in electrophilic reactions . Indole inhibits the enzymes chymotrypsin , lysozyme and tryptophanase .
proof
The indole test is used in the identification of bacteria . To do this, add a drop of dimethylaminobenzaldehyde ( Ehrlich reagent , Kovacs reagent ) to the MIO tube of the colorful series , which turns cherry red when tryptophan has been broken down into indole.
Indole derivatives
There are numerous indole derivatives that play a role in nature (example: tryptophan ), technology (example: indigo ) and pharmacology (example: indomethacin ).
Web links
Individual evidence
- ↑ Entry on INDOLE in the CosIng database of the EU Commission, accessed on June 30, 2020.
- ↑ a b entry on indole. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ a b c d e f Entry on indole in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Data sheet indole (PDF) from Merck , accessed on September 5, 2016.
- ↑ Baeyer, A .: About the reduction of aromatic compounds by means of zinc dust . In: Ann. . 140, 1866, p. 295. doi : 10.1002 / jlac.18661400306 .
- ↑ Baeyer, A .; Emmerling, A .: Synthesis of Indole . In: Chemical Reports . 2, 1869, p. 679. doi : 10.1002 / cber.186900201268 .
- ^ RB Van Order, HG Lindwall: Indole . In: Chem Rev.. . 30, 1942, pp. 69-96. doi : 10.1021 / cr60095a004 .