Chymotrypsin B
| Chymotrypsin B | ||
|---|---|---|

|
||
| Ribbon model of chymotrypsin according to PDB 4cha . The catalytic triad - aspartate-102, histidine-57, serine-195 - and a section of the inhibitor eglin C (purple) are highlighted | ||
| Properties of human protein | ||
| Mass / length primary structure | 241 = 13 + 131 + 97 AA | |
| Precursor | Chymotrypsinogen B (245 AA) | |
| Identifier | ||
| Gene name | CTRB1 | |
| External IDs | ||
| Drug information | ||
| ATC code | B06 AA04 , S01 KX01 | |
| Enzyme classification | ||
| EC, category | 3.4.21.1 , serine protease | |
| MEROPS | S01.152 | |
| Substrate | Tyr - + - Xaa, Trp - + - Xaa, Phe - + - Xaa, Leu - + - Xaa | |
| Occurrence | ||
| Homology family | Trypsin | |
| Parent taxon | Creature | |
Chymotrypsin B is a digestive enzyme that is very similar in structure to trypsin , but differs from it in particular in its milk-coagulating effect. The human chymotrypsins B and C belong to the serine proteases and are almost identical within the higher mammals . Several other chymotrypsins from marine life and insects have been identified.
Chymotrypsin B is produced in the pancreas in the form of an inactive zymogen precursor (chymotrypsinogen). Chymotrypsinogen is split into three subunits by trypsin in the small intestine , and the assembly of the subunits converts it to the active form (chymotrypsin).
history
Moses Kunitz (1887–1978) and John Howard Northrop produced chymotrypsin in crystalline form in 1935; In 1947, C. Jacobsen realized that there were various modifications .
In 1962 TL Shields used it for the therapy of herpes zoster and A. Rosanova also achieved success in 1967 in the treatment of herpes zoster, viral pneumonia and influenza .
Use in medicine
As an active ingredient, chymotrypsin is part of enzyme preparations that are used for thrombophlebitis and other inflammations.
It is also used locally in ophthalmology as a proteolytic .
The determination of chymotrypsin in the stool can provide information about various diseases of the pancreas, in particular exocrine pancreatic insufficiency . The determination of pancreatic elastase in the stool is considered to be more sensitive .
biochemistry
Chymotrypsin B preferably cleaves at peptide bonds whose carbonyl group comes from an aromatic amino acid ( L - tyrosine , L - tryptophan or L - phenylalanine ) or from L - leucine . It is one of the endopeptidases . Due to the mechanism of action with the catalytic triad of L - aspartate , L - histidine and L - serine , it is assigned to the serine proteases .
These three polar amino acids, which are highly conserved in serine proteases, are also found in trypsin and elastase .
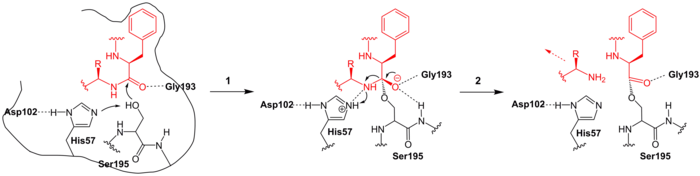
A likely enzymatic mechanism is shown in the figures below. Here, the polypeptide to be cleaved binds to chymotrypsin B, whereby the hydrophobic amino acid contained in the substrate (a phenylalanine , red in the figure ) reaches a binding pocket. This is also stabilized by a glycine (Gly193) through hydrogen bonds .
In the first reaction step ( 1 ), the catalytic serine (Ser195) attacks the peptide bond that is to be cleaved by nucleophilic attack. The histidine-57 (His-57) acts as a base because it removes a proton from Ser195. This creates a short-lived, tetrahedral transition state, in which the bond to the remaining substrate peptide is subsequently cleaved ( 2 ). This leaves the enzyme. The remaining acyl-enzyme intermediate, however, is stable and can also be isolated with the help of substrate analogues.
When water is stored (step 3 , blue), it reacts as a nucleophilic agent and attacks the carbonyl carbon of the intermediate. His57 acts as a base and takes up a proton from the water. A tetrahedral transition state forms again ( 4 ). This is short-lived ( 5 ). This releases the remaining polypeptide and as a result regenerates the Ser195. A new cycle can begin.
Individual evidence
- ^ A b c Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2nd, revised and expanded edition. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 2005, ISBN 978-3-8047-2113-5 , p. 115 .
- ↑ Olav Hagemann: Chymotrypsin in the stool. In: laborlexikon.de. Retrieved November 23, 2016 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules: An introduction to organic chemistry . Helvetica Chimica Acta / Wiley-VCH 2006; ISBN 978-3-906390-29-1 ; P. 453.
- ^ Reginald Garrett and Charles M. Grisham: Biochemistry . (International Student Edition). Thomsom Learning Inc .; 3rd edition 2005; ISBN 0-534-41020-0 ; P. 434ff.
Web links
- Jennifer McDowall / Interpro: Protein Of The Month: Trypsin and Chymotrypsin. (engl.)