Molar mass
| Physical size | |||||||
|---|---|---|---|---|---|---|---|
| Surname | Molar mass | ||||||
| Formula symbol | |||||||
|
|||||||
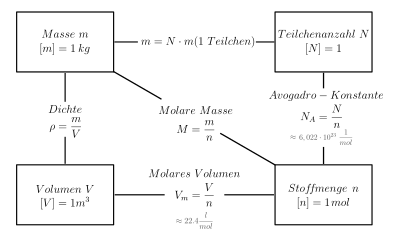
The molar mass (also out of date molar mass or molar weight ; unusual mass related to the amount of substance) of a substance is the mass per amount of substance or, in other words, the proportionality factor between mass and amount of substance :
- .
She is an intense quantity . The SI unit is kg / mol ; g / mol is common in chemistry.
The molar mass of a chemical compound is the sum of the molar masses of the chemical elements involved in the compound, multiplied by the respective stoichiometric factor . Stoichiometric factors are the numbers in the molecular formula of a molecule or, in the case of non-molecular compounds ( metals and ionic compounds ), the ratio formula .
The molar mass of an isotopically pure substance is constant. The numerical value of the molar mass in g / mol is the relative molecular mass and is equal to the numerical value of the molecular mass in the atomic mass unit (u or Dalton, if the compound consists of pure nuclide elements.)
definition
The individual symbols stand for the following quantities :
calculation
The molar mass of a compound can be calculated if you know its empirical formula : For each element, you can take the index number from the total formula - it appears in the total formula after the element symbol . For each element you have to z. For example, the molar mass can be taken from tables - its numerical value is equal to the relative atomic mass . Then the molar mass is obtained as the sum of the molar masses of the elements that make up the connection:
The molar mass of a compound is equal to the sum of the molar masses of the elements multiplied by their index numbers.
Example for water (H 2 O):
The molar masses of all compounds can be calculated from the molar masses of the chemical elements .
| element | Element icon | Atomic number | Molar mass |
|---|---|---|---|
| hydrogen | H | 1 | 1.00794 g / mol |
| carbon | C. | 6th | 12.0107 g / mol |
| oxygen | O | 8th | 15.9994 g / mol |
| connection | Molecular formula | Number of atoms | Molar mass |
| hydrogen | H 2 | 2 | 2.01588 g / mol |
| oxygen | O 2 | 2 | 31.9988 g / mol |
| water | H 2 O | 3 | 18.01528 g / mol |
| methane | CH 4 | 5 | 16.043 g / mol |
| Acetylsalicylic acid | C 9 H 8 O 4 | 21st | 180.16 g / mol |
determination
First determination
Avogadro's law was decisive for the first determination of the molar mass of molecules. In the gas state, the same number of molecules take up an almost identical volume at the same temperature. For simple molecules such as chlorine, hydrogen, hydrogen chloride, oxygen and water vapor, the ratios could be determined from weighing the gases after electrolysis. With the Bunsen method, molar masses of gases can be determined using the outflow times. For complex organic molecules, Avogadro's law was also used initially, in which the pure organic substances were evaporated and the volume of water displaced was determined. The method was first used by Joseph Louis Gay-Lussac and later improved by Victor Meyer . Another method was to measure the increase in the boiling point ( ebullioscopy ). A somewhat older process is that according to Dumas , in which the substances were also vaporized. For non-evaporable molecules, lowering of the freezing point ( cryoscopy ) or the osmotic pressure of solutions ( osmometry ) were used in the past . The latter method was developed by Jacobus Henricus van 't Hoff .
The first determination of the molar mass was based on the measurement of effects, the size of which depends only on the number of causing particles, but not on their mass ( colligative effects ).
Determination in the present
Mass spectrometry is an exact, sensitive measurement method . Here the relative molar mass results from the molecular peak, which is based on a calibration with a standard substance of known molar mass. In high-resolution mass spectrometry, the molar mass can be determined with four decimal places and the empirical formula can also be determined. It should be noted that the molar mass of a molecule - depending on its isotopic composition - can be subject to certain fluctuations. A technically less complex method is the electrophoretic determination of the molar mass , but this only allows an estimate. It plays an important role in the preparation of proteins , in restriction analyzes and other preparative methods.
Related sizes
- Mean molar mass
- Molar volume (molar volume)
- Molecular weight or molecular mass or molecular mass (formerly molecular weight)
Despite SI specifications to the contrary , the symbol M in the sense of molarity is still often used to indicate substance concentrations .
Web links
Individual evidence
- ↑ Science and Technology . The Brockhaus, Mannheim; Spectrum Academic Publishing House, Heidelberg, 2003.
- ↑ Since the revision of the International System of Units in 2019 , this equality no longer applies by definition, but approximately. The possible deviation, however, is of the order of 3 · 10 −10 and is irrelevant in practice. See atomic mass unit # relationship to molar mass .
- ^ S. Ebel, HJ Roth (Ed.): Lexicon of Pharmacy . Georg Thieme Verlag, 1987, ISBN 3-13-672201-9 , p. 445.