methane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | methane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 4 | |||||||||||||||
| Brief description |
colorless and odorless gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 16.04 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−182 ° C |
|||||||||||||||
| boiling point |
−162 ° C |
|||||||||||||||
| pK s value |
48 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Dipole moment |
0 |
|||||||||||||||
| Refractive index |
1,000444 (0 ° C, 101.325 kPa) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 10,000 ml m −3 or 6,700 mg m −3 |
|||||||||||||||
| Global warming potential |
28 (based on 100 years) |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−74.87 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methane is a chemical compound from the alkane group with the empirical formula CH 4 . The colorless, odorless, flammable gas occurs naturally and is the main component of natural gas . It serves as heating gas and is of great importance in the chemical industry as a starting product for technical syntheses.
As a greenhouse gas , methane has a high global warming potential and contributes to global warming at. In the earth's atmosphere , it is oxidized to carbon monoxide and eventually carbon dioxide. The carbon dioxide emissions during combustion are 2.74 kg CO 2 / kg methane.
The combustion takes place with a bluish-light flame in the presence of sufficient oxygen to carbon dioxide and water . Methane is insoluble in water and forms explosive mixtures with air. Because it is abundant in storage facilities, it is an attractive source of energy . The transport takes place through pipelines or as a frozen liquid by means of tankers. There are also deposits of methane hydrate on the sea floor , although the exact amount is unknown. The gas is also produced in considerable quantities by biological processes, for example in livestock farming .
Discovery and origin of name
The name methane is derived from Méthylène . This was the first name given by French chemists Jean-Baptiste Dumas and Eugène-Melchior Péligot in 1834 to the liquid that is now known as methanol . Its name is composed of the ancient Greek méthy ( ancient Greek μέθυ ) for intoxicating drink or wine and hylé ( ancient Greek ὕλη ) for wood. The German chemist August Wilhelm von Hofmann proposed a systematic nomenclature for the first time in an English publication in 1866 , according to which the name for gas was derived from methylene and should be called methane . This gave rise to the German form methane .
Methane was already known to alchemists in the Middle Ages as a component of putrefactive gases, also known as swamp air . In February 1659, the Englishman Thomas Shirley examined a spring with combustible water near Wigan . He was able to show that it was not the water, but a gas that rose from the bottom of the dammed water that caused the phenomenon. He suspected that the gas came from an underlying coal deposit and that it was mine gas . At that time nothing was known about the nature of the gas. In 1856, Marcellin Berthelot produced methane for the first time from carbon disulfide and hydrogen sulfide .
In ancient texts, methane was sometimes referred to as methyl hydrogen .
Occurrence and origin
| planet | proportion of |
|---|---|
| earth | 1.86 ppm |
| Mars | 0.0105 ppm |
| Jupiter | 3000 ± 1000 ppm |
| Saturn | 4500 ± 2000 ppm |
| Uranus | 23,000 ppm |
| Neptune | 15,000 ± 5000 ppm |
Earthly

It is assumed that, in addition to ammonia and water vapor, methane was a main component of the earth's primordial atmosphere .
Methane occurs in many ways and is constantly being re-formed on earth , e.g. B. in biological and geological processes ( serpentinization ). The methane concentration in the atmosphere has evolved from 1750 until 2018 from 0.73 to 1,869 ppm than doubled, reaching the highest since at least 800,000 years more.
Close to the surface
A large portion of the terrestrial methane is by microorganisms formed: When rotting of organic substances under airtight conditions in swamps or in sediment on the bottom of water bodies is formed swamp gas , a mixture of methane and carbon dioxide . Also biogas consists mainly of methane (about 60%) and carbon dioxide (about 35%), next to it still contains hydrogen , nitrogen and hydrogen sulfide .
The formation takes place biotically near the surface : on the one hand, anaerobic in the course of methanogenesis (from carbon dioxide and mostly acetate ). This is caused by special archaea , the methanogens . They use simple organic compounds like carbon dioxide or methanol and reduce them to methane, thereby generating energy. This process is called methanogenesis. For example, the formation of methane from CO 2 and hydrogen (H 2 ) under standard conditions at a pH value of seven releases about 131 kJ / mol of free enthalpy (Gibbs energy, ΔG 0 ' ):
- Methane and water are made from carbon dioxide and hydrogen
A small part of the biotic formation is based on the aerobic cleavage of methylphosphonates .
Underground

Below the surface of the earth, methane is formed in the deeper subsurface at high temperatures and pressures and is mostly released during volcanic activity . It is the main component of natural gas (85–98%), which occurs primarily as a companion to petroleum. The mine gas trapped in coal deposits also mainly contains methane. It can develop abiotic-thermal during the ripening process of coal (geochemical phase of coalification ), as well as from all types of kerogens and petroleum.
Methane that escapes from the sea floor is converted into solid methane hydrate due to the high pressure and low temperature . It is also known as “methane ice”. The carbon content of the world's methane hydrate deposits is estimated at 500–3000 gigatons . For comparison: the carbon content of the proven coal reserves is around 900 Gt.
Extracting methane hydrate could help solve earthly energy problems, but it is problematic. A particular problem is, for example, that a lot of methane would get into the earth's atmosphere during recovery and, as a very effective greenhouse gas , would contribute to further warming and thus further release of methane. In addition, the production of methane hydrate is dangerous. The first funding attempts are already underway. The consequences of overexploitation are largely unexplained; Researchers fear that the continental slopes , which are largely made up of methane ice (which could become unstable as a result of extraction), will slide down. Because of global warming and the associated warming of the sea, some researchers fear that methane hydrate will melt and evaporate. This would also bring methane into the earth's atmosphere as a greenhouse gas and intensify the anthropogenic greenhouse effect. The release of methane from thawing permafrost soil is another tipping element of climate change .
Emissions

Every year around 600 million tons of methane are emitted on earth; in Germany in 1994 about 833,000 tons. About 70% of the earth's microbial methane emissions are due to human activity . In the agriculture and the livestock methane is produced, 39% of these emissions go to the cattle back, 17% of the wet rice cultivation . The atmospheric dwell time is 9–15 years and is short compared to other greenhouse gases.
The increasing keeping of cattle, the frequent cultivation of wet rice and emissions of z. B. Landfill gases increase the greenhouse effect . The archaeal methane producers are mainly responsible for the constant formation of new methane. A domestic cattle z. B. emits around 150–250 liters of methane every day because methanogens are involved in the decomposition of cellulose in the cattle stomach .
New findings show that plants constantly produce methane and have thus always contributed to the methane content of the atmosphere. In 2006 the FAO attributed just under one fifth of anthropogenic (man-made) greenhouse gas emissions to the livestock sector, slightly more than the transport sector. Research is being conducted into reducing methane emissions from cows through feed additives (status 2007).
At the beginning of 2014, the research magazine Science reported after a meta-study of 200 studies that the US Environmental Protection Agency (EPA) had stated that methane gas emissions in the USA had been one to three quarters too low for 20 years. In the USA, 40 million tons more were emitted into the atmosphere annually than previously officially assumed, both from natural sources and from livestock, for example 88 million cattle in the country, as well as from leaks in conveyor systems and pipelines . So far it is unclear to what extent the incorrect information has an influence on the calculation models for the development of the global climate. In 2014, in the area around Four Corners, massive methane emissions from nearby coal mining sites were detected using satellite data. At an estimated 600,000 tonnes per year, emissions are greater than those of the entire UK oil, gas and coal industry. Studies suggest that methane emissions from coal mines have been greatly underestimated.
The fracking boom in the USA is accompanied by an increase in methane emissions. According to a study published in 2020, fracking plants emit twice as much methane as previously estimated.
According to two studies by the Global Carbon Project , more than 60 percent of methane emissions in 2017 came from the human economy.
Alien
The atmospheres of Mars , Titan , Jupiter , Saturn , Uranus , Neptune and Pluto also contain methane. Outside our solar system, methane was the first organic molecule to be detected on planets. In space, methane is therefore present in large quantities on planets, comets and moons.
Mars
In 2009 methane eruptions were reported on Mars ; in the atmosphere of Mars methane was detected, about 10.5 ppb . Since it cannot normally stay in the atmosphere and there is no evidence of meteorites as a source, it must have been newly formed on the planet, which can be an indication of life on Mars . The methane could also be of volcanic origin, for which no evidence has been found on Mars; However, in 2008 it was proven that the methane from the Lost City hydrothermal vents is of geochemical origin.
titanium
On Saturn's moon Titan there is almost the triple point of methane at −180 ° C and about 1.6 bar atmospheric pressure . Methane can therefore occur on this moon in all three aggregate states. There are clouds of methane, from which methane rains, which then flows through rivers, including the Vid Flumina , into methane lakes, where it evaporates again and thus forms a closed methane cycle (analogous to the water cycle on earth).
Liquid methane is transparent to radar rays, so the Cassini space probe was able to determine the depth of the Ligeia Mare lake to be 170 m.
There are probably icebergs made of methane / ethane on these lakes. These can only swim on the methane lakes if they contain at least 5% gaseous nitrogen . If the temperature drops only slightly, the nitrogen contracts so much that the ice sinks to the bottom. If the temperature rises again, the ground ice can rise again to the surface of the lake. At certain temperatures, surface and ground ice can occur simultaneously. For the Ontario Lacus , a lake near the south pole of Titan, the heavier ethane was found to be the main component .
Outside the solar system
In March 2008 methane gas was first found outside of our solar system, on the ( Exoplanet HD 189733 b of the Hot Jupiter type ).
Extraction and presentation
There are two methods of manufacturing aluminum carbide; they are mostly only used in the laboratory. The synthesis from carbon monoxide is of particular importance. Only the source of the hydrogen is a problem in this synthesis.
-
- Aluminum carbide reacts with water to form aluminum hydroxide and methane when heated.
-
- Aluminum carbide reacts with hydrochloric acid to form aluminum chloride and methane.
-
- Sodium acetate is heated together with sodium hydroxide to produce sodium carbonate and methane.
-
- Carbon monoxide reacts with hydrogen to form methane and water.
-
- Carbon dioxide reacts with hydrogen to form methane and water.
This reaction was discovered in the 19th century by the French and Nobel Prize winner Paul Sabatier and is therefore called the Sabatier process .
- Carbon dioxide reacts with hydrogen to form methane and water.
Today, a lot of methane is also produced as a fuel in biogas plants . Methane can also be obtained through wood gasification . The methanation after previous water electrolysis is the basic principle for the production of wind or solar gas , which is ascribed increasing importance in the field of renewable energies .
properties
Physical Properties
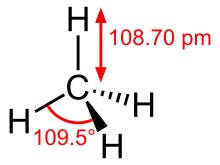
Methane melts at −182.6 ° C and boils at −161.7 ° C. Due to the non-polar properties, it is barely soluble in water, ethanol and diethyl ether , however, it dissolves well. Heat of fusion and heat of vaporization are 1.1 kJ / mol and 8.17 kJ / mol, but these are very low values compared to metals . The calorific value H i is 35.89 MJ · m −3 or 50.013 MJ kg −1 . The standard entropy is 188 J mol −1 K −1 , the heat capacity 35.69 J mol −1 K −1 . The triple point of methane is 90.67 K and 0.117 bar, the critical point is 190.56 K and 45.96 bar.
Solid methane exists in several modifications , nine different are currently known. When methane is cooled at normal pressure, methane I is produced. This is a cubic crystallizing substance, space group Fm 3 m (space group no. 225) . The positions of the hydrogen atoms are not fixed; H. the methane molecules can rotate freely. Therefore it is a plastic crystal .
The colorless and odorless gas has a lower density than air, so it rises into the higher layers of the earth's atmosphere . There it acts as a greenhouse gas , and it is 20 to 30 times more effective than carbon dioxide, but it occurs in much smaller quantities than this in the atmosphere. There it reacts with oxygen to form carbon dioxide and water. This process is slow, the half-life is estimated to be 12 years.
The UN numbers of compressed and frozen methane are 1971 and 1972, respectively. Methane is in the standard 50 liter steel bottle or in vehicle tanks (often with carbon fiber reinforced epoxy resin over aluminum liner) compressed to 200 bar (as CNG) in gaseous form. The ship transport in large quantities takes place in almost pressureless membrane tanks, but liquefied at about −160 ° C (LNG). Ships with tubular and spherical tanks transport natural gas at increased pressure and temperature.
Chemical properties
Methane is the simplest alkane and the simplest hydrocarbon , the empirical formula is CH 4 , the C – H bonds point into the corners of a tetrahedron . It is flammable and burns in the air with a bluish, non-sooting flame . It can react explosively with oxygen or chlorine , which requires initial ignition (supply of activation energy ) or catalysis . In the chlorination arise chloromethane , dichloromethane , chloroform and carbon tetrachloride . In the case of oxidation, on the other hand, the molecule is completely torn apart. The reaction of a methane molecule with two oxygen molecules results in two water molecules and one carbon dioxide molecule. Methyl compounds such as methane are derived from methane . B. methanol and the methyl halides and the longer-chain alkanes .
Reactions with oxygen
With oxygen , methane is different reactions one, depending on how much oxygen is available for the reaction. Complete combustion of the methane with optimal energy yield is only possible if there is a sufficient supply of oxygen .
If the oxygen supply is insufficient, however, by-products such as carbon monoxide (CO) and carbon ( soot ) are formed. Furthermore, the useful energy is lower in this case.
More reactions
Besides oxygen , methane enters into a wide variety of other reactions. Many of them are very important for the chemical industry as the products are of great technical importance.
- Methane reacts with sulfur at 700 ° C and with aluminum oxide catalysis to form carbon disulfide and hydrogen sulfide .
- Methane reacts with ammonia and oxygen on a platinum catalyst to form hydrogen cyanide and water.
- Methane reacts photochemically (light-induced) with halogens to form methyl halides and hydrogen halides , here for example with chlorine .
- Methane reacts at 1400 ° C and water vapor to ethyne and hydrogen .
- Methane reacts at 800 ° C on contact with nickel with water to form carbon monoxide and hydrogen.
use
Methane is mainly used as a heating gas to generate heat and to operate engines through combustion.
In addition to methane from other sources, biogas ( biomethane ) with a methane content of around 50 to 70% is obtained from manure , liquid manure , sewage sludge or organic waste for this purpose . In the past, methane was obtained by pyrolysis of wood , which produces wood gas ( wood gasification ). Wood gas, which contains methane among other things, was used to power civilian automobiles due to the lack of oil in World War II . The heating wood gasifiers were mostly built outside. Today, raw biogas from wastewater treatment plants is often converted directly into electricity using combustion engines.
Methane is an important starting product for technical syntheses of hydrogen , methanol , ethyne , hydrocyanic acid , carbon disulfide and methyl halides . It serves as the starting point for many other organic compounds .
The problem with using methane as an alternative fuel in engines is the methane slip, approx. 2% of the methane is not burned and is released into the atmosphere as an environmentally harmful greenhouse gas. Methane is therefore more harmful to the climate than diesel. Only in some 2-stroke engines should this problem not occur.
Other meaning
Risk of explosion
Methane forms explosive mixtures with a volume fraction between 4.4 and 16.5 percent in air. The unnoticed leakage of natural gas repeatedly results in serious gas explosions. The dreaded mine gas explosions in coal mines ( firedamp ) can also be traced back to methane-air mixtures. Methane is highly flammable, the flash point is −188 ° C, the ignition temperature is 595 ° C. Methane tanks should be kept in well-ventilated places and kept away from sources of ignition and measures should be taken to prevent electrostatic charging. In order to increase the density, methane is stored under high pressure in gas bottles at 200 bar. In tankers, methane is liquefied at almost zero pressure and is transported at about −160 ° C.
Greenhouse gas

Methane is a potent greenhouse gas : Its global warming potential is based on a period of 100 years, 28 times higher than the same weight of carbon dioxide ; According to a more recent study, this factor is 33 if interactions with atmospheric aerosols are taken into account. In relation to a period of 20 years, this factor even rises to 84, so the horizon is more realistic about the current demand and the disintegration of the molecules, as it was also called in the IPCC report AR5. Methane contributes around 20% to the anthropogenic greenhouse effect. There is far more methane in the earth's atmosphere than ever during the last 650,000 years; the methane concentrations rose annually between 2000 and 2006 by about 0.5 ppb , since 2006 at a rate more than ten times higher. Meanwhile, methane emissions in Germany were halved between 1990 and 2009. This rapid increase could be related to the production of shale gas through hydraulic fracturing (fracking). It is believed that methane, as a greenhouse gas, caused the largest mass extinction of the Phanerozoic about 252 million years ago ; the increase in its concentration in the earth's atmosphere in modern times is one of the aspects of the Anthropocene .
The release of methane from permafrost and the sea floor is a possible consequence and another cause of global warming.
Undetected leaks in refineries and the transportation of oil and gas through pipelines emit significant amounts of methane; the International Energy Agency (IEA) in Paris estimated this amount at the end of 2017 to be around 75 million tons per year and thus 1.7% of the total output.
In an oxygen-containing atmosphere, methane is slowly oxidized, especially by hydroxyl radicals . The average lifetime in the atmosphere is around 12.4 years.
A study by researchers at the University of Rochester published in February 2020 sees strong evidence that around ten times less methane escapes naturally from geological sources than previously assumed. Conversely, the promotion of fossil energies (oil, gas and coal) has a significantly higher proportion of methane emissions.
Biological importance
Methane is oxidized by certain bacteria in water and soils with oxygen (O 2 ) to carbon dioxide and water. This conversion is exergonic and the bacteria use it as an energy source, which is why they are among the methanotrophic microorganisms .
toxicology
Methane is stored in liquid form at low temperatures because this can increase the density enormously. For this reason, frostbite can easily occur when this cooled methane escapes. Methane is non-toxic , the ingestion of methane can lead to increased breathing rate ( hyperventilation ) and increased heart rate, it can cause short-term low blood pressure , numbness in the extremities, drowsiness, mental confusion and memory loss , all caused by a lack of oxygen. However, methane does not cause permanent damage. If symptoms occur, leave the affected area and take a deep breath; if symptoms do not go away, the affected person should be taken to hospital.
proof
Methane can be detected using infrared spectroscopy. Infrared spectroscopic detection is established for the detection of extraterrestrial occurrences. Methane can also be detected and quantified using gas chromatography and gas chromatography with mass spectrometry coupling . In mining previously to warn of firedamp about the used various qualitative detection methods, Davy lamp with Flammsieb , canaries or firedamp whistle .
literature
Books
- Christian Felske: Minimization of residual gas emissions from municipal waste landfills through methane oxidation in landfill cover layers . (= Forum Urban Water Management and Waste Management, University of Essen. Issue 20). Aachen 2003, ISBN 3-8322-2168-9 . (At the same time dissertation at the University of Duisburg-Essen 2003)
- Peter Pfeifer, Roland Reichelt (Eds.): H 2 O & Co. Organic Chemistry. Oldenbourg, Munich 2003, ISBN 3-486-16032-X . (Extra chapter on methane and information also on the other alkanes)
- Christiane Werth: On methane activation in molten salts . In: Reports from chemistry . Shaker, Aachen 2003, ISBN 3-8322-2597-8 . (At the same time dissertation at the Rheinisch-Westfälische Technische Hochschule Aachen 2003)
Magazine articles
- Plants - a forgotten source of methane. In: Mining. Issue 1/2007, pp. 7–8. ( Digital version, PDF file, 72.2 kB )
Web links
"Climate killer methane: The misunderstood danger" , report Munich, June 25, 2019, video, BR-Mediathek
Individual evidence
- ↑ a b c d e f g h i Entry for CAS no. 74-82-8 in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ DH Ripin, DA Evans: pKa's of Inorganic and Oxo-Acids. (PDF) Retrieved July 15, 2014 .
- ↑ S. Budavari et al. a .: The Merck Index , 12th edition. MERCK & CO., 1996, p. 1018
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Gases, pp. 10-254.
- ↑ Entry on methanes in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 74-82-8 or methane ), accessed on September 15, 2019.
- ↑ a b c G. Myhre u. a .: Anthropogenic and Natural Radiative Forcing. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge / New York 2013, p. 731, (PDF)
- ↑ MW Chase, Jr .: NIST-JANAF Thermochemical Tables . (= Journal of physical and chemical reference data / Monograph. 9). 4th edition, 1998, ISBN 1-56396-831-2 , pp. 1-1951
- ↑ George A. Olah, Alain Goeppert, GK Surya Prakash: Beyond oil and gas: the methanol economy. Verlag Wiley-VCH, 2009, ISBN 978-3-527-32422-4 (Note: The 3rd (expanded and updated) edition was published in 2018; ISBN 978-3527338030 ).
- ^ August Wilhelm von Hofmann: On the action of trichloride of phosphorus on the salts of the aromatic monoamines in Proceedings of the Royal Society of London , Volume 15, pp. 55-62; Footnotes: pp. 57–58 ( online )
- ↑ Thomas Shirley: The Description of a Well, and Earth in Lanchashire, Taking Fire by a Candle Approached to It in Philosophical Transactions of the Royal Society , Volume 2, 1667, pp. 482-484 ( online )
- ^ A b Giora Proskurowski, Martin D. Lilley, Jeffery S. Seewald, Gretchen L. Früh-Green, Eric J. Olson, John E. Lupton, Shean P. Sylva, Deborah S. Kelley: Abiogenic hydrocarbon production at Lost City Hydrothermal Field. In: Science , Volume 319, 2008, pp. 604-607
- ↑ William Martin: Everything has a beginning, including evolution: hydrothermal springs and the origin of life (PDF; 945 kB). In: Biology of Our Time. 3/2009 (39), pp. 166-173. doi: 10.1002 / biuz.200910391
- ↑ Federal Agency for Civic Education : Methane in the Atmosphere (CH 4 ) , accessed on April 28, 2019
- ↑ Federal Environment Agency: Atmospheric Greenhouse Gas Concentrations - Methane (article from February 19, 2019), accessed on April 28, 2019
- ^ Robin McKie: Sharp rise in methane levels threatens world climate targets . In: The Observer . February 17, 2019, ISSN 0029-7712 ( theguardian.com [accessed July 14, 2019]).
- ↑ Greenhouse gas concentrations in atmosphere reach yet another high. In: WMO. November 25, 2019, accessed November 27, 2019 .
- ↑ U. Deppenmeier, V. Müller: Life close to the thermodynamic limit: how methanogenic archaea conserve energy. In: Results Probl Cell Differ. Volume 45, 2008, pp. 123-152. PMID 17713742 ; doi : 10.1007 / 400_2006_026
- ^ David M. Karl, Lucas Beversdorf u. a .: Aerobic production of methane in the sea . In: Nature Geoscience , 1, 2008, pp. 473-478, doi: 10.1038 / ngeo234
- ↑ Siddhesh S. Kamat, Howard J. Williams et al. a .: The catalytic mechanism for aerobic formation of methane by bacteria . In: Nature , 497, 2013, pp. 132-136, doi: 10.1038 / nature12061
- ^ MJ Hunt: Petroleum geochemistry and geology . 2nd edition, WH Freeman and Company, New York 1995
- ^ B. Buffet, D. Archer: Global inventory of methane clathrate: Sensitivity to changes in the deep ocean . In: Earth and Planetary Science Letters . Vol 227, 2004, pp. 185–199, (PDF; 610 kB)
- ↑ AV Milkov: Global estimates of hydrate-bound gas in marine sediments: how much is really out there? In: Earth Science Reviews , Vol. 66, 2004, pp. 183-197
- ↑ BP.com, June 2006: Quantifying energy - BP Statistical Review of World Energy , (PDF)
- ↑ Methane Thrower Permafrost - Wissenschaft.de . In: Wissenschaft.de . March 20, 2018 ( Wissenschaft.de [accessed March 7, 2019]).
- ↑ Frontiers 2018/19: Emerging Issues of Environmental Concern. Retrieved March 7, 2019 .
- ↑ Entry on methane. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ Plants with bad breath. On: Wissenschaft.de from January 12, 2006.
- ↑ Livestock's Long Shadow - Environmental Issues and Options (en), FAO 2006, Rome, ( short version , en)
- ↑ Garlic 'may cut cow flatulence' , BBC , July 10, 2007
- ↑ Seaweed in Cow Feed Reduces Methane Emissions Almost Entirely , accessed May 11, 2018
- ↑ The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. February 9, 2016, accessed May 11, 2018 .
- ↑ Breanna M. Roque, Joan K. Salwen, Rob Kinley, Ermias Kebreab: Inclusion of Asparagopsis armata in lactating dairy cows' diet reduces enteric methane emission by over 50 percent. In: Journal of Cleaner Production. 234, 2019, p. 132, doi : 10.1016 / j.jclepro.2019.06.193 .
- ↑ Silke Hasselmann: USA have given the emission of the greenhouse gas methane too low. ( Memento from February 27, 2014 in the Internet Archive ) on: Deutschlandfunk . February 14, 2014, (February 20, 2014)
- ^ Jillian Ambrose: Methane emissions from coalmines could stoke climate crisis - study . In: The Guardian . November 15, 2019, ISSN 0261-3077 ( theguardian.com [accessed November 15, 2019]).
- ↑ mbe: Satellite image of the week: Huge methane leak discovered in the USA. In: spiegel.de. October 11, 2014, accessed October 11, 2014 .
- ↑ Josh Gabbatiss: Coal mines emit more methane than oil-and-gas sector, study finds. Carbon Brief, March 24, 2020, accessed on March 29, 2020 .
- ↑ Robert W. Howarth: Ideas and perspectives: is shale gas a major driver of recent increase in global atmospheric methane? In: Biogeosciences . August 14, 2019 ( biogeosciences.net [accessed August 20, 2019]).
- ↑ Fracking boom tied to methane spike in Earth's atmosphere. In: National Geographic. August 15, 2019, accessed August 20, 2019 .
- ^ Science Advances : Quantifying methane emissions from the largest oil-producing basin in the United States from space
- ↑ cbsnews April 25, 2020: Huge amounts of methane leaking from US oil fields, study shows
- ↑ Susanne Schwarz: Let the mad cow disease , in: taz, July 15, 2020.
- ^ Astronomers Detect First Organic Molecule on an Exoplanet. from: jpl.nasa.gov (March 19, 2008), accessed March 20, 2008
- ↑ Michael J. Mumma et al. a .: Strong Release of Methane on Mars in Northern Summer 2003 . In: Science , January 15, 2009
- ↑ Kenneth Chang: NASA Rover on Mars Detects Puff of Gas That Hints at Possibility of Life . In: The New York Times . June 22, 2019, ISSN 0362-4331 ( nytimes.com [accessed July 16, 2019]).
- ↑ NASA's Cassini Spacecraft Reveals Clues About Saturn Moon . From: jpl.nasa.gov , December 12, 2013, accessed December 27, 2013
- ↑ Ralph-Mirko Richter: Ice floes on the surface of the Titan Lakes? In: Raumfahrer.net . January 13, 2013, accessed June 11, 2013
- ↑ Cassini Suggests Icing on a Lake . From: jpl.nasa.gov , January 8, 2013, accessed June 11, 2013
- ↑ R. Bini, G. Pratesi: High-pressure infrared study of solid methane: Phase diagram up to 30 GPa . In: Physical Review . B, 55 (22), 1997, pp. 14800-14809
- ↑ Visualization of the crystal structure of methane as a solid
- ↑ Standard enthalpy of reaction for the combustion of methane and various petroleum products, cf. P. 3 ff. In: Herbert Mayr: Lecture 9: Petroleum processing . (PDF file; 190 kB). LMU Munich: Physical-Organic Chemistry, 2006
- ↑ Ivan Ernest: binding, structure and reaction mechanism in organic chemistry . Springer-Verlag, 1972, ISBN 3-211-81060-9 , pp. 297-306
- ↑ taz from February 7, 2020: New fuel for ships. Pipi for the climate
- ↑ DT Shindell, G. Faluvegi, DM Koch, GA Schmidt, N. Unger, SE Bauer: Improved attribution of climate forcing to emissions. In: Science. 326, No. 5953, 2009, pp. 716-718
- ^ S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, KB Averyt, M. Tignor and HL Miller (eds.): Ipcc.ch: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (PDF, 3.9 MB). In: IPCC, 2007: Summary for Policymakers . In: Climate Change 2007 , Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ↑ Environmental Research Letters , doi : 10.1088 / 1748-9326 / 11/12/120207 . According to: deutschlandfunk.de , research news , reports , December 12, 2016: Climate change: Methane concentrations in the atmosphere are currently increasing unusually quickly (June 20, 2017)
- ↑ Greenhouse gas emissions in Germany. Federal Environment Agency, April 25, 2019, accessed on February 29, 2020 .
- ↑ Robert W. Howarth: Ideas and perspectives: is shale gas a major driver of recent increase in global atmospheric methane ?. In: Biogeosciences. 16, 2019, p. 3033, doi : 10.5194 / bg-16-3033-2019 .
- ↑ Reuters: Scientists shocked by Arctic permafrost thawing 70 years sooner than predicted . In: The Guardian . June 18, 2019, ISSN 0261-3077 ( theguardian.com [accessed July 2, 2019]).
- ↑ Volker Mrasek: Methane losses - leaks in the oil and gas industry. In: Deutschlandfunk. Germany Radio, December 6, 2017 accessed on 8 December 2017 .
- ↑ greenhouse gases. Federal Environment Agency, June 6, 2019, accessed on February 29, 2020 .
- ↑ Benjamin Hmiel et al .: Preindustrial 14CH4 indicates greater anthropogenic fossil CH4 emissions (February 19, 2020); German-language report on spektrum.de (19 February 2020)
- ↑ K. Kawara, B. Gregory, T. Yamamoto, H. Shibai: Infrared spectroscopic observation of methane in Comet P / Halley. In: Astronomy and Astrophysics. 207, 1, 1988, pp. 174-181, bibcode : 1988A & A ... 207..174K
- ↑ Lee Marotta, Denis Yates: Methane, Ethylene, and Ethane in Water by Headspace-Gas Chromatography (HS-GC) with Flame Ionization Detection (FID). Retrieved October 26, 2014 .
- ^ Fritz Haber: About firedamp display . In: The natural sciences . 1, 1913, pp. 1049-1051, doi: 10.1007 / BF01492997