carbon
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Carbon, C, 6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Non-metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 14 , 2 , p | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | black (graphite) colorless (diamond) yellow-brown (Lonsdaleite) dark gray (chaoite) |
||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-44-0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-153-3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.321 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.087% | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 12.011 (12.0096-12.0116) u | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 70 (67) pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 76 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 170 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ He ] 2 s 2 2 p 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 11.260 288 0 (11) eV ≈ 1 086.45 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 24.383 154 (16) eV ≈ 2 352.62 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 47.88778 (25) eV ≈ 4 620.47 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 64.49352 (19) eV ≈ 6 222.68 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 392.090 515 (25) eV ≈ 37 831 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 3 (including graphite (G) and diamond (D)) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | G: hexagonal D: face-centered cubic |
||||||||||||||||||||||||||||||||||||||||||||||||
| density | G: 2.26 g / cm 3 D: 3.51 g / cm 3 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | G: 0.5 D: 10 |
||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism |
diamagnetic (D: Χ m = −2.2 10 −5 ; G: up to −4.5 10 −4 ) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Sublimation point | 3915 K (3642 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | G: 5.31 · 10 −6 m 3 / mol D: 3.42 · 10 −6 m 3 · mol −1 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | Sublimation : 715 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | D: 18350 m · s −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | G: 709 J / (kg K) D: 427 J kg −1 K −1 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 4.81 eV | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, 0, 1, 2, 3, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.55 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||
Carbon (from Urm. Kul-a-, kul-ō (n) - , coal ') or carbon (from Latin carbō , charcoal', Latinized carboneum or carbonium ) is a chemical element with the element symbol C and the atomic number 6. In the periodic table it is in the fourth main group or the 14th IUPAC group or carbon group and the second period .
It occurs in nature in a dignified (pure) form ( diamond , graphite , chaoite ) as well as chemically bound (e.g. in the form of carbides , carbonates , carbon dioxide , crude oil , natural gas and coal ). Due to its special electron configuration (half-filled L-shell) it has the ability to form complex molecules and has the greatest variety of chemical compounds of all chemical elements . This property makes carbon and its compounds the basis of life on earth.
Occurrence

Carbon is an essential element of the biosphere ; in all living beings - after oxygen ( water ) - it is the most important element in terms of weight. All living tissue is made up of (organic) carbon compounds.
Geologically, on the other hand, carbon is not one of the most common elements, because the mass fraction of carbon in the earth's crust is only 0.027%.
In inanimate nature, carbon is found both in elemental form ( diamond , graphite ) and in compounds. The main locations of diamond are Africa (above all South Africa and the Democratic Republic of the Congo) and Russia. Diamonds are often found in volcanic rocks such as kimberlite . Graphite is relatively rare in carbon-rich metamorphic rocks . The most important deposits are in India and China.
More than half of the carbon is in the form of inorganic carbonate rock (approx. 2.8 · 10 16 t). Carbonate rocks are widespread and sometimes form mountains. A well-known example of carbonate mountains are the Dolomites in Italy. The most important carbonate minerals are calcium carbonate (modifications: limestone , chalk , marble ) CaCO 3 , calcium magnesium carbonate ( dolomite ) CaCO 3 · MgCO 3 , iron (II) carbonate ( iron spar ) FeCO 3 and zinc carbonate ( zinc spar ) ZnCO 3 .
Well-known carbon deposits are the fossil raw materials coal , crude oil and natural gas . These are not pure carbon compounds, but mixtures of many different organic compounds . They were created by converting vegetable (coal) and animal (oil, natural gas) remains under high pressure. Important coal deposits are in the USA, China and Russia, a well-known German in the Ruhr area . The most important oil reserves are on the Arabian Peninsula (Iraq, Saudi Arabia). There are other important oil deposits in the Gulf of Mexico and the North Sea. Little is known about solid methane hydrate in the deep sea.
Carbon continues to exist in the air as carbon dioxide (carbon dioxide for short). Carbon dioxide is produced when carbon-containing compounds are burned, when breathing, and volcanically, and is used by photosynthesis in plants. CO 2 is also dissolved in water (approx. 0.01% mass fraction in the sea). As of 2015, there were around 830 billion tons of carbon in the atmosphere. Since the burning of fossil fuels since the beginning of industrialization has been adding long-term bound CO 2 to the material flows in the environment , the proportion of the composition of the air increases gradually. In 2015 the proportion was 400 ppm or 0.04%; an increase of approx. 120 ppm compared to the pre-industrial value of 280 ppm. In total, around 530 billion tons of carbon have been released by fossil fuels since the beginning of industrialization, almost half of which remained in the atmosphere and a good quarter of each was absorbed by the oceans and terrestrial ecosystems.
In terms of quantity, the majority of the carbon is stored in the rock shell ( lithosphere ). All other deposits make up only about 1/1000 of the total carbon in terms of quantity.
properties
Physical Properties
Carbon occurs in several allotropic modifications . All carbon-based solids can be traced back to the two basic types diamond and graphite.
In diamond, carbon is covalently bonded in three dimensions . Diamond is an insulator and is transparent. It is the hardest known natural material and is used as an abrasive.
In graphite, the covalent bond within the basal planes is stronger than that in diamond, while the planes are loosely bound by van der Waals forces . The free π electrons are responsible for the deep black color, the easy cleavage and the high conductivity along the basal planes. Graphite is used as a high-temperature-resistant sealing material and lubricant as well as a base material for pencil leads .
Contrary to popular belief, however, the well-known lubricating properties of graphite are not a property of graphite per se, but are only found in the presence of traces of moisture. In a vacuum or very dry atmosphere, the coefficient of friction of graphite increases dramatically.
At normal pressure and temperatures below 4000 K, graphite is the thermodynamically stable modification of carbon (see phase diagram). Because of the high activation energy, diamond is also stable at room temperature and only changes noticeably above 500 ° C to graphite. Conversely, the transformation from graphite to diamond requires a pressure of at least 20,000 bar (2 GPa). For a sufficiently fast reaction, the temperature should be above 1500 ° C., at a pressure of 60,000 bar according to the phase diagram.
Carbon has the highest temperature resistance of all known materials. It sublimates at normal pressure at 3915 K (3642 ° C) without previously losing strength. The triple point is (10.8 ± 0.2) MPa and (4600 ± 300) K.
Carbon is diamagnetic. Pyrolytically deposited graphite has a large anisotropy in the magnetic susceptibility (parallel: = −85 · 10 −6 ; perpendicular: = −450 · 10 −6 ), diamond, on the other hand, is isotropic ( = −22 · 10 −6 ).
In its various modifications, carbon shows very different properties. Carbon is the hardest element: as a crystalline diamond, the absolute maximum value of 90 GPa is reached on the Knoop hardness scale . In the form of graphite, carbon is the third softest element after rubidium and cesium with 0.12 GPa. Carbon also has the highest thermal conductivity, which is well over 2000 W / (m · K) at room temperature.
Chemical properties
Molecular carbon has low chemical activity due to its stable configuration . It can react if the atom is supplied with additional energy and the electrons in the outer shell have to break off. At this moment the valence of the element becomes 4, and for this reason carbon in compounds has an oxidation state of +2, +4 and −4. All reactions of carbon with metals and non-metals take place at high temperatures . This element can be both an oxidizing agent and a reducing agent . The reducing properties of carbon are strong, so the element is used in the metal industry .
The ability of carbon to undergo chemical reactions depends on factors such as the reaction temperature , the allotropic modification and the degree of dispersion. It does not react with alkalis and acids and very rarely with halogens . One of the main properties of carbon is the element's ability to form long chains between itself. The chains close cyclically and branches are formed. In this way, millions of organic compounds are created . These compounds can also contain other elements: oxygen , nitrogen , sulfur , phosphorus , halogens or metals .
Atomic model of carbon
The model of atomic and molecular orbitals illustrates how the different manifestations of carbon come about.
Carbon has six electrons. According to the shell model, two electrons occupy the inner 1s shell. The 2s level of the second shell also accepts two electrons, two more the 2px and 2py levels. Only the four outer electrons of the second shell appear chemically. The probability of the electrons being in an s level is spherical. In a p level it is anisotropic. The electrons populate an hourglass-shaped space, one half hourglass to the left and right of the center along the x-axis, if one imagines the atom placed in the center of a Cartesian coordinate system . The py and pz orbital (according to the y and z axes) are perpendicular to this .
Diamond structure ( sp 3 )
The 2s level can hybridize with the 3 2p levels and form 4 energetically equivalent sp 3 orbitals. This can be explained clearly in such a way that one of the s-electrons is lifted into the previously empty p-orbital and the orbital energies of all four orbitals of the second level are equalized. The newly emerging orbitals have an elongated, asymmetrical teardrop shape. If the shapes of the p orbitals were arranged point-symmetrically to the center, they now appear club-like enlarged in one direction. The picture shows the main lobes, the side lobes have been left out for the sake of clarity. The four sp 3 orbitals are oriented symmetrically in space with the greatest possible distance from one another, they point to the corners of an imaginary tetrahedron .
If the sp 3 orbitals of atoms overlap , they can form firm covalent bonds , which then reflect the tetrahedral structure. They form the basic structure of the diamond lattice (see crystal structure there.)
Graphite structure ( sp 2 )
If only 2 of the 3 p orbitals are involved in the hybridization, the so-called sp 2 orbitals arise . The sp 2 orbitals align two-dimensionally (as a surface or plane); Above and below this surface the remaining p orbital forms an orbital lobe each. For example, if the p orbital is perpendicular to the xy plane , the sp 2 orbitals are trigonal in the xy plane. They have the same angle of 120 ° to each other. The picture on the left illustrates the situation. The unhybridized p orbital is omitted for the sake of clarity.
sp 2 carbon atoms can form covalent bonds with one another, which then lie in one plane. Their structure is trigonal; this is the basic structure of the planar planes of graphite (see crystal lattice structure there). The remaining p orbitals also interact with one another. They form the pi bonds with significantly lower binding energies than the sigma bonds of the sp 2 or sp 3 orbitals and form a so-called electron gas in the form of atomic core independent (“delocalized”) pi electrons above and below the sigma bond plane .
Chemically, one speaks of a double bond . The notation C = C neglects the different character of both bonds. The binding energy of the diamond-like tetrahedral sp 3 single bond 'C – C' is 350 kJ / mol, that of the graphite-like trigonal sp 2 double bond C = C is only 260 kJ / mol higher. In a hexagonal carbon ring with six carbon atoms, the pi bond is stabilized by delocalizing the electrons within the ring (for more on this see benzene ).
Triple bond ( sp 1 )
If only one p orbital hybridizes with the s orbital, two linearly arranged pi binding lobes result. If we orientate them along the x-axis, the remaining p orbitals lie on the y- and z-axes. Two sp -hybridized atoms can form a carbon triple bond. One example is the gas ethyne (acetylene) HC ≡ CH . While sp 3 bonds form three-dimensional structures and sp 2 two -dimensional structures , sp 1 bonds form at most one-dimensional (linear) chains, such as H – C≡C – C≡C – H.
Forms of carbon
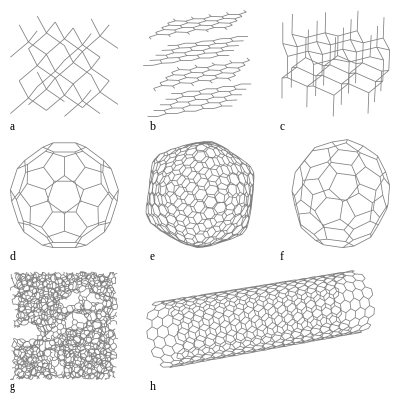
Elemental carbon exists in three modifications based on the bond structures sp 3 and sp 2 : diamond, graphite and fullerene.
In addition to these three modifications, there are other different forms of elemental carbon.
Modifications
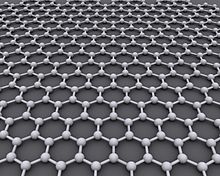
graphite
The sp 2 -covalently hexagonally bonded carbon atoms form high-strength levels. The levels are only loosely bound by Van der Waals forces . Macroscopically, cleavage dominates along the planar planes. Because the planes are so thin, their extraordinary strength is not apparent with graphite.
Because of this structure, graphite behaves very anisotropically : along the crystal planes graphite is thermally and electrically very conductive, heat conduction or charge transfer from crystal plane to crystal plane, on the other hand, works relatively poorly.
diamond
The sp 3 -covalently tetragonally bonded carbon atoms have no free electrons. The material is an insulator with a band gap of 5.45 eV that does not absorb visible light. The addition of foreign atoms creates states in the band gap and thus changes the electrical and optical properties. The yellowish tone of many natural diamonds can be traced back to nitrogen, while boron-doped diamonds look bluish and are semiconducting. In the absence of air, the diamond turns into graphite at temperatures around 1500 ° C. It burns to carbon dioxide at around 700–800 ° C.
Under normal conditions (1 bar, 25 ° C) diamond is generally considered to be the metastable form of carbon. However, based on recent research, this is no longer certain because
- the thermodynamic stability is only extrapolated to low PT conditions,
- In equilibrium studies, the influence of the environment - small traces of impurities that are below the current detection limit can already have drastic effects on the equilibrium position of a reaction - was / is not taken into account and ultimately
- Experiments by Chinese scientists show that in a reaction between metallic sodium and magnesium carbonate, carbon and diamond coexist in a stable manner.
Lonsdaleit
Lonsdaleite, also known as the hexagonal diamond , is a very rare modification of the diamond. It occurs when graphite is converted into diamond by shock events, i.e. high pressure and high temperature such as impact events . The hexagonal character of the crystal structure is retained, but in contrast to graphite, each carbon atom is covalently bound to four others .
Chaoite
Chaoite is a very rare modification that crystallizes in the hexagonal crystal system similar to graphite, but with different lattice parameters and a slightly different crystal structure. Similar to Lonsdaleit, it is created by shock metamorphosis in graphitic gneiss.
Fullerenes
A regular hexagonal honeycomb pattern, as it is formed by the carbon atoms in the basal planes of graphite, is planar. If you replace some hexagons with pentagons, you get curved surfaces, which "roll up" to form closed bodies with certain relative arrangements of the five and six rings. Such structures are implemented in the fullerenes . The sp 2 bonds are no longer in one plane, but form a spatially closed structure. The smallest possible structure consists only of pentagons and requires 20 carbon atoms, the corresponding body is a pentagon dodecahedron . This simplest fullerene has so far only been detected by mass spectrometry . One of the most stable fullerenes consists of 60 carbon atoms and, apart from hexagons, only contains pentagons that have no edge in common with any other pentagon. The resulting pattern ( truncated icosahedron , an Archimedean body ) resembles the pattern on an (old-fashioned) soccer ball . It is named Buckminster fullerene in honor of Richard Buckminster Fuller . The molecular “spheres” of the fullerenes are bound to one another via relatively weak van der Waals interactions , similar to the basal planes in graphite. In the meantime, a number of fullerenes of different sizes have been isolated and in some cases also crystallized; they can therefore be considered a real modification (s). Fullerenes are believed to be found in all soot, for example in the soot over candle flames.
Cyclo [18] carbon
Cyclo [18] carbon is a cyclic modification of carbon discovered in 2019 with the empirical formula C 18 , which is stable at low temperatures close to absolute zero.
Other forms of carbon
Amorphous carbon
In amorphous carbon (aC) the atoms are networked without long-range order. The material can be produced with almost any sp 2 : sp 3 hybridization ratios, with the material properties transitioning smoothly from those of graphite to those of diamond. In this case, the term diamond-like coating or diamond-like carbon (DLC) is often used in industry. If the proportion of sp 3 hybridization exceeds 70%, one speaks of tetrahedral amorphous carbon (ta-C). This material is characterized by high electrical resistance, extreme hardness and optical transparency. Production can be carried out using PVD or PECVD methods. The material is deposited as a layer (amorphous carbon layer ).
Carbon fibers
Carbon fibers consist of graphite-like sp 2 -bonded carbon. Isotropic fibers behave similarly to polycrystalline graphite and have only low strengths. Fiber mats and bundles are used for thermal seals. By stretching during manufacture, it is possible to align the basal planes along the fiber axis. High-strength fibers are obtained with properties that approximate the theoretical values of graphite along the basal planes. Anisotropic carbon fibers are light, extremely stiff and strong and are used in composite materials .
Vitreous carbon
Glassy carbon ( "glassy carbon") is combined with those of a high-technological material of pure carbon, the vitreous and ceramic properties of the graphite. In contrast to graphite, vitreous carbon has a fullerene-like microstructure. This results in a wide variety of positive material properties. For example, the conductivity is lower than that of graphite.
Graph
A graphite basal plane of sp 2 -hybridized carbon is called a graph . The thin layers are obtained by chemically splitting graphite. Embedded in plastics, it is suitable as a starting material for new composite materials or for investigating two-dimensional crystals, and research is also being carried out into electronics applications .
Activated carbon
Careful graphitization of organic materials such as coconut shells leads to a porous carbon. The cavities are connected to one another like a sponge and form a very large inner surface. Activated carbon filters low concentrations of dissolved substances from liquids and can adsorb gases.
soot
Soot is also made of graphite-based carbon. The purer the carbon black, the more clearly the properties of graphite emerge. Lamp or candle soot is heavily contaminated with organic compounds that prevent the formation of larger graphite associations.
Carbon nanotubes
Another form of carbon is sp 2 -hybridized carbon atoms arranged in a cylindrical manner . Its geometry is created from a planar layer of graphite that is rolled up into a cylinder. The resulting tube can also be twisted, which changes the electrical properties. Several single-walled tubes can lie concentrically inside one another, so that one speaks of multi-walled carbon nanotubes (MWCNT), in contrast to single-walled carbon nanotubes (SWCNT).
Carbon nanobuds
Carbon nanobuds combine the properties of carbon nanotubes and fullerenes.
Carbon nanofoam
Carbon nanofoam , an airgel , is a randomly oriented, network-like arrangement of carbon-graphite layers. It is similar to vitreous carbon, only with significantly larger interconnected cavities. Their average diameter is six to nine nanometers .
A distinction must be made between this and carbon airgel , which consists of nanoparticles that have grown together. Its density is between 200 and 1000 kg / m 3 .
Aerographite
Aerographite consists of a network of porous carbon tubes and is one of the lightest solids in the world with a density of 0.2 milligrams per cubic centimeter. Aerographite can be compressed by up to 95% and pulled apart again into its original shape.
Non-graphitic carbon
“Non-graphitic carbon consists of layers of hexagonally arranged, sp 2 -hybridized carbon atoms. These layers are stacked almost parallel without any three-dimensional long-range order. ”This material consists of stacks of graphene layers that are twisted and shifted against each other . This arrangement is also known as turbostratic. The distance between the layers can differ significantly from the layer distance found in graphite. The microstructure analysis of the material is possible using WAXS , among other things , but due to the wide and overlapping maxima caused by the significant disorder, standard methods such as the Scherrer equation cannot be used.
Carbine (pearl necklaces)
In 2016, it was possible to synthesize straight chains called carbine , which consist of more than 6000 atoms, within double-walled nanotubes .
Q-carbon
Q-Carbon is a man-made diamond-like allotropic form of carbon that is described as ferromagnetic and harder than diamond.
links
Carbon is the element that can form the most compounds of all elements after hydrogen (hydrogen comes first because most carbon compounds also contain hydrogen). The peculiarities of carbon are to form chains and rings with itself and other elements as well as double and triple bonds with the participation of π orbitals. Due to its moderate electronegativity, it binds well to both more electropositive and more electronegative elements. All oxidation states from −IV to + IV occur naturally in inorganic or organic compounds.
With a few exceptions, carbon compounds are traditionally included in organic chemistry ; this is sometimes referred to as the chemistry of carbon. Organic chemistry includes more compounds than all inorganic chemistry because of the ability of carbon to form long chains and covalent bonds with other atoms . Also, the biochemistry is a part of the organic carbon chemistry. The simplest organic compounds include the alkanes methane and ethane .
Only relatively few carbon compounds are traditionally placed next to inorganic compounds, among them the most important in terms of quantity are oxygen compounds:
- Carbides , carbon element compounds of the type E x C y , in which the carbon is the more electronegative reactant. Many metals can form carbides, some of which are very hard and are used for cutting tools (e.g. tungsten carbide ).
- Carbon monoxide CO is a very toxic gas that has a strong reducing effect and plays an important role in metal smelting (e.g. iron ).
- Carbon dioxide CO 2 is a by many combustion processes arising greenhouse . It is exhaled by most living things and used by plants in photosynthesis . Carbon dioxide is now around 0.04% of the atmosphere, in the pre-industrial era the proportion was 0.028%.
- Carbonic acid H 2 CO 3 is a metastable product of water and CO 2 dissolved in the water ; a moderately strong acid, but with regard to the constant conversion between carbonic acid and dissolved CO 2, it is usually combined with CO 2 .
- Suboxides such as tricarbon dioxide (malonic anhydride, C 3 O 2 ), tetra carbon dioxide (C 4 O 2 ), pentacarbon dioxide (C 5 O 2 ), oxalic anhydride (C 4 O 6 ) and mellitic anhydride (C 12 O 9 ).
- Hydrogen carbonates or bicarbonates E + HCO 3 - , the best-known representative of which is sodium hydrogen carbonate , used as a leavening agent , among other things .
- Carbonates E 2+ CO 3 2− are the divalent salts of carbonic acid. The two best-known carbonates are sodium carbonate , common name soda, an important raw material for glass production, and calcium carbonate , from which z. B. mussels , snails build their shells and deposit the hard corals . The calcium carbonate formed by them and by other processes over long periods of time now forms entire mountains (see: Limestone ). Calcium carbonate is still an important building material.
- Carbon-sulfur compounds, the best known of which is carbon disulfide ( carbon disulfide , CS 2 ), a very toxic liquid.
- Carbon-nitrogen compounds such as cyanides , the best-known representative of which is potassium cyanide, a very strong poison that blocks breathing . Many other cyanides are similarly toxic.
Isotopes
A total of 15 isotopes between 8 C and 23 C of carbon are known. Two of these, the isotopes 12 C and 13 C, are stable and occur in nature. The isotope with the greater proportion of the natural isotope composition is 12 C with 98.93%, 13 C has a proportion of 1.07%. The longest-lived unstable isotopes are 11 C, which changes to 11 B with a half-life of 20.364 minutes under β + radiation , and 14 C, which decays to 14 N with a half-life of 5730 years under beta decay. All other isotopes only have short half-lives of seconds or milliseconds.
According to the definition, 12 C is the reference point for the unit of atomic mass . 13 C can be detected in NMR spectroscopic investigations because, unlike 12 C, it has a magnetic moment. The ratio of these two isotopes is called Δ13C and is used in geochemistry , paleoclimatology and paleoceanography .
14 C results from the reaction of 14 N with cosmic rays . Living organisms that take part in the carbon cycle show the same proportion of 14 C in relation to the total amount of carbon they contain as the atmosphere. After the end of metabolism, for example after a tree has been felled, this proportion gradually decreases due to the radioactive decay. The determination of the proportion of 14 C to the total carbon content therefore allows the age of objects made of organic material to be determined, the radiocarbon method , which is mainly used in archeology .
CO 2 obtained from natural gas or petroleum has lower levels of 14 C than CO 2 from the air, where the 14 C isotope is continuously reproduced. 14 C can therefore be used as a kind of tracer to determine the path or content of molecules based on petroleum in plants with the help of scintillation spectrometers . For example, the CO 2 transport in the respiratory chain .
The short-lived isotope 11 C is used as a PET nuclide . To do this, it is generated on a cyclotron and converted into radiopharmaceuticals such as [ 11 C] choline using suitable chemical processes .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 861-918.
- NN Greenwood, A. Earnshaw: Chemistry of the Elements. 1st edition. VCH Verlagsgesellschaft, Weinheim 1988, ISBN 3-527-26169-9 , pp. 327-419.
Web links
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (carbon) , unless otherwise stated .
- ↑ The standard value recommended by IUPAC is given, since the isotopic composition of this element can vary locally, the mass range given in brackets results for the mean atomic weight. See: Michael E. Wieser, Tyler B. Coplen: Atomic weights of the elements 2009 (IUPAC Technical Report). In: Pure and Applied Chemistry , 2010, p. 1 ( doi: 10.1351 / PAC-REP-10-09-14 ).
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e entry on carbon in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on carbon at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 864.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b M. D. Simon, AK Geim: Diamagnetic levitation: Flying frogs and floating magnets. In: Journal of Applied Physics . 87, 2000, pp. 6200-6204 ( doi: 10.1063 / 1.372654 ).
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-8.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-135.
- ↑ a b Entry on carbon in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ^ A b Theodore L. Brown, H. Eugene Le May, Bruce E. Bursten: Chemistry The central science . MZ Pearson Studium Deutschland GmbH, Munich 2007, ISBN 978-3-8273-7191-1 , p. 1123 .
- ↑ Markus Reichstein: Universally and Everywhere. The terrestrial carbon cycle in the climate system. In: Jochem Marotzke , Martin Stratmann (Hrsg.): The future of the climate. New insights, new challenges. A report from the Max Planck Society. Beck, Munich 2015, ISBN 978-3-406-66968-2 , pp. 125–127.
- ↑ Bing K. Yen, Birgit E. Schwickert: Origin of low-friction behavior in graphite investigated by surface x-ray diffraction. (PDF; 215 kB), May 2004.
- ^ A. Greenville Whittaker: The controversial carbon solid-liquid-vapor triple point . In: Nature . tape 276 , 1978, pp. 695-696 , doi : 10.1038 / 276695a0 .
- ↑ JM Zazula: On Graphite Transformations at High Temperature and Pressure Induced by Absorption of the LHC Beam. (PDF) CERN, 1997, accessed June 6, 2009 .
- ↑ GIT laboratory journal. Issue 9/2013, p. 596, according to Jürgen Quadbeck-Seeger (Ed.): Chemie Rekorde. Wiley-VCH.
- ↑ MEL Science: Properties and characteristics of carbon, and its reactions with oxygen
- ^ MA Carpenter: Thermodynamics of phase transitions in minerals: a macroscopic approach. In: Stability of Minerals. Chapman & Hall, London 1992.
- ^ E. Salje: Phase transitions in ferroelastic and coelastic crystals. Cambridge University Press, Cambridge 1990.
- ↑ Katharina Kaiser, Lorel M. Scriven, Fabian Schulz, Przemyslaw Gawel, Leo Gross, Harry L. Anderson: An sp-hybridized molecular carbon allotrope, cyclo [18] carbon . In: Science . 2019, p. eaay1914 , doi : 10.1126 / science.aay1914 .
- ^ Translation from E. Fitzer, K.-H. Kochling, HP Boehm, H. Marsh: Recommended Terminology for the Description of Carbon as a Solid (IUPAC Recommendations 1995). In: Pure and Applied Chemistry , 1995, 67, pp. 473-506 ( doi: 10.1351 / pac199567030473 ): "Non-graphitic carbons consists of layers of hexagonally arranged sp 2- carbon atoms that are stacked nearly parallel without any three-dimensional long-range order. "
- ↑ Chemistry: record length: carbon as a pearl necklace. on: orf.at , April 4, 2016, accessed April 4, 2016.
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi : 10.1088 / 1674-1137 / 41/3/030001 ( full text ).