silver
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Silver, Ag, 47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 11 , 5 , d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | glossy white, metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-22-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-131-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.301 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC code | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.079 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 107.8682 (2) et al | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 160 (165) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 145 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 172 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Kr ] 4d 10 5 s 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.576 234 (25) eV ≈ 731 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 21st.4844 (9) eV ≈ 2 072.93 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 34.8 (3) eV ≈ 3 358 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 49.0 (1.7 eV) ≈ 4 728 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 65.0 (1.9) eV ≈ 6 272 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 10.49 g cm −3 (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 to 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −2.4 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1234.93 K (961.78 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 2483 K (2210 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 10.27 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 254 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 11.3 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2600 m s −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 235 (25 ° C, constant pressure) J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 4.26 eV | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 61.35 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 430 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +1 , +2, +3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | 0.7991 V (Ag + + e - → Ag) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.93 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAK |
Switzerland: 0.1 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Silver is a chemical element with the element symbol Ag and the atomic number 47. It is one of the transition metals . In the periodic table it is in the 5th period and the 1st subgroup (group 11) or copper group. The element symbol Ag derived from the Latin word a r g entum for "silver" from. Silver is one of the precious metals .
It is a soft, easily deformable ( ductile ) heavy metal with the highest electrical conductivity of all elements in the unmodified state (carbon in the form of graphene has another higher conductivity) and the highest thermal conductivity of all metals . Only superfluids and undisturbed crystalline forms of carbon ( diamond , graphene and graphite close to graphite , carbon nanotubes ) and boron nitride have better thermal conductivity.
etymology
The word “silver” ( Old High German silabar, silbar and similar forms) is derived from the common Germanic root * silubra- , just like the names in other Germanic languages (such as English silver ). The Basque has taken the Germanic word: zilar . There are related names in the Baltic languages ( Lithuanian sidabras ) and the Slavic languages ( Russian серебро serebro , Croatian srebro ).
The philology of the 19th century brought a variety of theories out on the word origin. The connection established by Victor Hehn in 1870 with the legendary land of Alybē ( Ἀλύβη ) described in Homer's Iliad must remain speculation. The word could come from an oriental language, derived from the Semitic root ṢRP (cf. Akkadian ṣarāpu , “ennoble, alloy”).
In other Indo-European languages , the word for silver goes back to the root * arg , such as ἄργυρος argyros in ancient Greek and argentum in Latin . Argentina was named for the silver that Europeans hoped to find there; it is the only country named after a chemical element. It is more common to name an element after a country, e.g. B. Francium , Germanium and Polonium .
history
Silver has been used by humans since around the 5th millennium BC. . Chr processed. It was used, for example, by the Assyrians , the Goths , the Greeks , the Romans , the Egyptians and the Teutons . At times it was considered more valuable than gold . Most of the silver came from the mines in Laurion , about 50 kilometers south of Athens . Silver was known as moon metal to the ancient Egyptians.
In the Middle Ages and the early modern period, silver ore deposits were found in Central Europe in the Harz Mountains ( Goslar ), in Waldeck-Frankenberg ( Frankenberg , Goddelsheim , Dorfitter , Thalitter ), on the Donnersberg ( Imsbach ), in the Thuringian Forest ( Ohrdruf ), in Saxony ( Freiberg and others Ore Mountains , especially Jáchymov ), discovered in the southern Black Forest ( Schauinsland , Belchen , Münstertal , Feldberg ), Bohemia ( Kutná Hora ) and Slovakia . Abundant silver deposits are also known from Kongsberg (Norway).
The largest silver producer in the Middle Ages was Schwaz . Up to 80% of the silver at that time came from the Schwazer miners' tunnels .
The Spaniards later brought large amounts of silver from Latin America , including from the legendary Potosí mine , to Europe. Japan was also a silver exporter in the 16th century. Due to the increased supply, the silver value in the Old World fell.
Since gold was mainly used as a currency metal after 1870, silver lost its economic importance more and more. The value ratio fell from 1:14 to 1: 100 for a while, later it rose again a little. In March 2018 it was around 1:81. The supply of silver depends on the development of consumption and production of other metals.
In the middle of the 19th century, stainless steel was developed, which, due to its user-friendliness and attractive price, made its way into silver applications after the First World War , such as serving plates, cutlery, candlesticks and kitchen utensils. In the opposite direction, the field of photography and photochemistry using silver salts developed broadly throughout the 20th century, but has lost much of its importance since the end of the 1990s in the course of the switch to digital imaging technology.
The greatest silver speculation is considered to be the bubble in the silver market from the mid-1970s to 1980, which is particularly associated with the brothers Nelson Bunker Hunt and William Herbert Hunt, the silver speculation of the Hunt brothers .
Silver as a mineral and varieties
Silver has a share of about 0.079 ppm in the earth's crust . It is around 20 times more common than gold and around 700 times less common than copper . In nature it occurs dignified , that is, elementary; mostly in the form of grains, more rarely of larger nuggets , thin plates and sheets or as a wiry, branched network ( dendrite ) or as thin silver wires in hydrothermally formed ore veins and in the area of the cementation zone .
Natural occurrences of native silver were already known before the International Mineralogical Association (IMA) was founded. Silver is therefore recognized as a so-called grandfathered mineral as an independent type of mineral.
According to the Strunz system of minerals (9th edition) , silver is classified under system no. "1.AA.05" (elements - metals and intermetallic compounds - copper cupalite family - copper group) or in the outdated 8th edition classified under I / A.01 (copper series). The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. "01.01.01.02" (gold group).
In addition to solid silver, of which more than 5500 locations have been documented so far (as of 2018), it is mainly found in sulfidic minerals. The most important sulfidic silver ores include acanthite ( silver luster ) Ag 2 S with a silver content of around 87% and stromeyerite ( copper silver luster ) CuAgS with around 53% silver. However, the mineral with the highest silver content of a maximum of 99% is the rarely occurring allargentum . Also rarely occurring silver minerals include the Chlorargyrite (obsolete Hornerz or Silberhornerz ) AgCl and Miargyrit ( Silver stibnite ) AgSbS 2 . A total of 167 silver minerals including native silver are known to date (as of 2018).
In addition to these silver ores, there are also so-called silver-containing ores, which usually only contain small amounts of silver (0.01–1%). These are often galena (PbS) and chalcopyrite (CuFeS 2 ). For this reason, silver is often extracted as a by-product in lead or copper production.
An than Kongsbergit designated silver amalgam having a mercury content of about 5% is used as a variety attributed to the silver. Kongsbergit is known from a little more than 30 localities so far.
As Arquerit a Silbervarietät (is silver amalgam ) designated with a mercury content of 10 to 15%.
Chilenite is a silver variety containing bismuth .
A silver variety with a content between 10 and 30% gold is known as Küstelite and has so far (as of 2011) been detected at around 60 sites.
It has been known since the 18th century that artificially ( anthropogenically ) produced silver wires, mostly in the form of silver curls, can be created by heating acanthite or by smelting silver ores . Especially in the last few decades there have been repeated reports in the specialist literature about the artificial production of silver curls on acanthite levels.
Occurrence and extraction
The most important silver deposits are in North America ( Mexico , the USA and Canada ) and in South America ( Peru , Bolivia ). With just under 20% of global production, Peru was the world's largest silver producer from 2003 to 2009 and was overtaken by Mexico in 2010. In 2017 Mexico produced the most silver worldwide with 6110 t, followed by Peru with 4300 t.
Most silver is obtained from silver ores, which are often found together with lead, copper and zinc ores as sulfides or oxides . Important sites of silver in solid form were: Freiberg in the Erzgebirge ; Schwaz (Tyrol) ; Kongsberg / Norway (there also large crystals); Sankt Andreasberg in the Harz Mountains; Keweenaw Peninsula / USA (there with also native copper as "halfbreed"); Batopilas / Mexico; Mansfeld copper slate district ( Eisleben , Sangerhausen ; mostly silver sheets; also used as petrifying material for fossils).
Between the beginning of the 20th century and the end of the Second World War , the amount of silver mined annually fluctuated, but on average it remained constant. From the end of the war until today it has more than doubled.
The Polish company KGHM is the most important silver company in the EU and the third largest in the world , with an average of 1,200 tons of annual production .
According to a study by the Rhine-Westphalian Institute for Economic Research , the Fraunhofer Institute for Systems and Innovation Research and the Federal Institute for Geosciences and Raw Materials , the global reach of silver resources is only 29 years. Thus, a shortage of silver is to be expected in the next few decades. However, more and more silver is also being recycled , which protects the known deposits. Based on the US Geological Survey data from January 2019, silver has a static range of 21 years in relation to 2017 .
As with other precious metals, the reprocessing of silver-containing materials plays an important role in recycling , for example photo paper , X-ray films , developer and fixer baths, electronic scrap and batteries .
| rank | country | Delivery rates (in t ) |
rank | country | Delivery rates (in t ) |
|
|---|---|---|---|---|---|---|
| 1 |
|
6110 | 7th |
|
1200 | |
| 2 |
|
4300 | 8th |
|
1120 | |
| 3 |
|
3500 | 9 |
|
1030 | |
| 4th |
|
1290 | 10 |
|
1020 | |
| 5 |
|
1260 | rest | 4770 | ||
| 6th |
|
1240 | total | 26800 | ||
Extraction and presentation
Extraction from silver ores
20% of the silver is extracted from silver ores. The silver is usually extracted from these by cyanide leaching with the help of a 0.1% sodium cyanide solution. To do this, the ore is first finely ground into a sludge . The sodium cyanide solution is then added. Good ventilation is important here as oxygen is required for the process .
With the addition of sodium cyanide both go elemental silver and silver ores (Ag 2 S, AgCl) as dicyanoargentate (I) [Ag (CN) 2 ] - in solution:
- ,
- ,
- .
Since the reaction of sodium cyanide with silver sulfide is in equilibrium, the sodium sulfide must be removed either by oxidation with oxygen or by precipitation (e.g. as lead sulfide). Then the nobler silver is precipitated with zinc - similar to gold mining :
- .
The precipitated raw silver ( factory silver ) is filtered off and further purified (see refining ).
Extraction from lead ores
In the extraction of lead ores, e.g. B. from lead luster , after roasting and reducing the so-called raw lead or work lead (more detailed information on lead extraction in the article lead ). This usually still contains a proportion of silver (between 0.01 and 1%). In the next step, the precious metal is removed and this valuable by-product is obtained.
To obtain it, the silver must first be separated from most of the lead. This is done using the Parkesierens process (after Alexander Parkes , who invented this process in 1842). The process is based on the different solubility of silver and lead in zinc . At temperatures up to 400 ° C, lead (liquid) and zinc (solid) are practically immiscible. First, zinc is added to the molten lead at temperatures> 400 ° C. The mixture is then cooled. Since silver is easily soluble in molten zinc, it changes into the zinc phase. The zinc melt then solidifies as a so-called zinc foam (zinc-silver mixed crystals). This allows the silver to be separated from most of the lead. This zinc foam is also known as arm lead . It is then heated to the melting point of the lead (327 ° C) so that part of the lead melts and can be removed. The remaining zinc-lead-silver melt is then heated to the boiling point of zinc (908 ° C) and the zinc is distilled off. The product obtained in this way is called rich lead and contains around 8–12% silver.
In order to enrich the silver, the so-called forcing work ( refining ) is carried out. For this, the rich lead is in a hearth furnace melted. A current of air is then passed through the melt. The lead oxidizes to lead oxide , while the precious silver remains unchanged. The lead oxide is continuously drained and so the lead is gradually removed. If the lead content of the raffinate has dropped so far that a matt lead oxide layer no longer forms on the surface of the molten metal, the last thin layer of oxide tears open and the shiny silver underneath becomes visible, this is known as the silver look . The alloy then available is called Blicksilber and consists of over 95% silver.
Extraction from copper ores
Silver is also found in copper ores . During copper production, the silver - along with other precious metals - is obtained in what is known as the anode sludge . This is first freed from the majority of the remaining copper with sulfuric acid and air. It is then melted in an oxidizing manner in the furnace, whereby the base metals contained go into the slag and can be removed.
Refining
Raw silver is cleaned electrolytically using the Moebius process . For this purpose, the raw silver is connected to an electrolysis cell as an anode. A fine silver sheet serves as the cathode and nitric acid silver nitrate solution as the electrolyte .
The process corresponds to the electrolytic cleaning of copper. During the electrolysis, silver and all the less noble components of the raw silver (e.g. copper or lead) are oxidized and go into solution. More noble parts like gold and platinum cannot be oxidized and fall under the electrode. There they form the anode sludge, which is an important source of gold and other precious metals . Only silver is now deposited on the cathode. This very pure silver is known as electrolyte or fine silver.
properties
Physical Properties
Silver is a shiny white precious metal . The metal crystallizes in the face-centered cubic crystal system . Under normal pressure, the melting temperature is 961 ° C and the boiling temperature is 2212 ° C. However, silver already has a significant vapor pressure above 700 ° C, i.e. still in the solid state . It boils with the formation of a single-atom, blue vapor. The precious metal has a density of 10.49 g / cm³ (at 20 ° C) and therefore belongs to the heavy metals like all precious metals.
Silver has a metallic sheen. Fresh, uncorroded (cut) surfaces of silver show the highest light reflection properties of all metals, freshly deposited silver reflects over 99.5% of the visible light. As the “whitest” of all utility metals, it is therefore also used to manufacture mirrors. The line color is a grayish white. As the grain size decreases, the color becomes darker and is black in the case of photographically finely distributed silver crystals. The reflection spectrum shows a pronounced plasma edge in the near UV .
Of all metals, silver is the best conductor of heat and electricity . Because of its elasticity and softness ( Mohs hardness of 2.5-4) it can be hammered into the finest, blue-green shimmering foils with a thickness of only 0.002 to 0.003 mm or into thin wires with a length of only 0.1 to 1 g (Filigree wire).
In the molten state, pure silver easily dissolves 20 times the volume of oxygen from the air, which escapes again when the melt solidifies and the already solidified surface bursts ( cracks ). Even slightly alloyed silver does not show this property.
Chemical properties

Silver is a precious metal with a normal potential of +0.7991 V. For this reason it is relatively inert. It does not react with the oxygen in the air, even at higher temperatures. Since the air contains traces of hydrogen sulfide H 2 S, silver surfaces turn black over time, since elemental silver forms silver sulfide (Ag 2 S) with hydrogen sulfide in the presence of atmospheric oxygen :
- .
Silver only dissolves in oxidizing acids such as nitric acid . It is not soluble in non-oxidizing acids. In the presence of oxygen, it also dissolves in cyanide solutions through the formation of a very stable silver cyanide complex, which greatly shifts the electrochemical potential. Silver only dissolves in concentrated sulfuric and nitric acid at elevated temperatures, as it is passivated by silver nitrate and sulfate . Silver is stable to molten alkali hydroxides such as sodium hydroxide . In the laboratory, silver crucibles instead of porcelain or platinum crucibles are therefore used for these melts.
Biological-medicinal properties
In finely divided form , silver has a bactericidal effect, i.e. slightly toxic , which is due to the large reactive surface area due to the sufficient formation of soluble silver ions. In the living organism, however, silver ions are usually quickly bound to sulfur and are eliminated from the material cycle as dark, poorly soluble silver sulfide . The effect depends on the surface. This is used in medicine for wound dressings as well as for invasive devices (e.g. endotracheal tubes). As a rule, silver is therefore used for bactericidal purposes in medical products as a coating or in colloidal form, and increasingly also nanosilver. Silver ions are used as disinfectants and as therapeutic agents in wound therapy. They can reversibly inhibit silver-sensitive pathogens after a relatively long exposure time, and can also have a bacteriostatic or even bactericidal (i.e. killing) effect. One speaks here of the oligodynamic effect. In some cases chlorine compounds are added to increase the low effectiveness of the silver.
Different mechanisms of action are used:
- Blocking enzymes and preventing their vital transport functions in the cell,
- Impairment of cell structure strength,
- Damage to the membrane structure.
The effects described can lead to cell death.
In addition to argyria , an irreversible slate-gray discoloration of the skin and mucous membranes, increased silver accumulation in the body can also lead to taste and smell disorders and cerebral seizures . Silver accumulates in the skin, the liver , the kidneys , the cornea of the eyes, in the gums , in the mucous membranes , nails and the spleen .
The therapeutic use of colloidal silver is controversial , which has been coming back into the public eye for several years and is being marketed via the Internet and other channels. It is mainly touted as a universal antibiotic and is said to be able to cure other ailments. There are no scientific studies on the effectiveness. Already comparable with a standard antibiotic effect is at peroral strongly to question administration. According to the American environmental protection agency EPA, very small orally ingested amounts of up to 5 micrograms of silver per kilogram of body weight and day should not lead to poisoning.
In 2014, silver was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Silver uptake was driven by concerns about high (aggregated) tonnage, other hazard-related concerns and widespread use. The re-evaluation took place from 2014 and was carried out by the Netherlands . A final report was then published.
Mythological properties
In many fairy tales and sagas, silver is considered the only metal that is able to kill werewolves and other mythological beings, which is also often taken up in modern fantasy novels and films.
use
Silver price
The price of silver is determined on the open market. This has been happening at London Bullion Market since the 17th century . The introduction of the silver fixing in London in 1897 marked the beginning of the market structure. In 1987 the London Bullion Market Association (LBMA) was founded. Three LBMA members participate in the silver fixing every working day chaired by the ScotiaMocatta . Other members of the silver fixing are Deutsche Bank AG London and HSBC Bank USA NA London Branch.
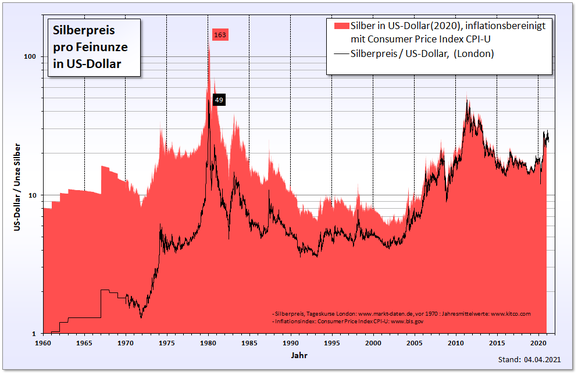
In the 1970s, which led silver speculation of the brothers Hunt to a record level in the silver price . Together with wealthy business people from Saudi Arabia, they bought huge amounts of silver and silver contracts on the commodity futures exchanges and tried to dominate the silver market. On January 18, 1980, the silver fixing at the London Bullion Market reached a record high of 49.45 US dollars per troy ounce . The silver price reached its next record more than 31 years later, on April 25, 2011, when the troy ounce of silver was traded at $ 49.80 in Hong Kong. According to the United States Department of Labor's inflation calculator, $ 49.45 in 1980 was $ 134.99 in 2011. It will therefore be a long time before the 1980 price is exceeded, taking inflation into account.
For standardized silver trading on commodity exchanges , “ XAG ” was assigned as a separate currency code according to ISO 4217 . It denotes the price of one troy ounce of silver (31.1 grams).
Currency and investment
The most important use in the past was the production of silver coins as a means of payment. In ancient times and in the Middle Ages, only silver, gold and copper or bronze were used for coins . The coin value largely corresponded to the metal value ( Kurant coin ). In Germany were 1871 silver coins ( taler prevalent), the currency was by silver covered ( Silver Standard ). After 1871 the silver standard was replaced by the gold standard . The reason for the use of these precious metals was the high retention of value (rarity) and stable value of silver and gold. Only in modern times are coins made of other metals such as iron, nickel or zinc, but their metal value is lower and does not correspond to the value imprinted on them ( dividing coin ). As a coin metal, silver is mostly only used for investment , commemorative and special coins.
Especially in times of economic crisis - such as B. from 2007 - in addition to gold, the precious metal silver has established itself as one of the most important forms of investment in various forms due to its price and value stability. B. silver bars, silver jewelry or silver coins. In the context of currency crises there has been a silver ban several times in history since ancient times (see gold ban ).
Economy and sport
In addition to gold and precious stones (e.g. diamonds ), silver is an important material for the manufacture of jewelry and has been used for centuries for exquisite and valuable cutlery ( table silver ) and sacred utensils . Silver stamps (maker's mark, city mark, tax stamp and other hallmarks) provide information about the origin of the item. In the case of jewelry, tools and bars, the silver content, if specified, can be read from the fineness stamp .
Silver medals are awarded in many sports competitions, e.g. B. at the Olympic Games , awarded as a sign of achieving second place. The Olympic gold medal also consists of 92.5% silver and is only gilded with 6 g of pure gold. Awards are also often referred to as “silver” in other areas. Examples are Silver Bear , Silver Stylus , Silver Shoe, and Silver Bay Leaf .
It is also very popular for musical instruments , because its density gives it a nice, warm tone, is easy to work with B. replaced the sensitive wood in the flute .
Silver has the highest electrical conductivity of all metals, a high thermal conductivity and a pronounced optical reflectivity . This makes it ideal for applications in electrics , electronics and optics . The reflectivity of glass mirrors is based on the chemical silver plating of glass panes. This principle is also used in the production of Christmas tree decorations, optics and light or heat reflectors. A suspension of silver powder in adhesives turns them into electrically (and thermally) conductive adhesives.
The blackening of the silver halides as a result of their decay through light and development is used in photo paper . It formed the basis of photography from around 1850 until the spread of digital technology .
Silver alloys (with copper , zinc , tin , nickel , indium etc.) are used in electrical engineering and soldering technology as solder alloys (so-called hard soldering ), contact materials (e.g. in relays) and conductive material (e.g. as capacitor coatings). Silver alloys are also used in dental technology and in the decorative sector.
Silver dishes and utensils always give off some silver to food and drinks when they are used, which can be noticeable in an unpleasant metal taste, especially with some drinks (wine). To avoid this, silver drinking vessels are often gilded on the inside. Silver tarnished by silver sulfide is either polished or chemically reduced (see silver care ).
Silver in medical and medical-related applications
Materials or coating processes use the antibacterial effect of silver in medical products and other applications in the form of silver coatings, as colloidal silver , nanosilver or in the form of silver threads. Examples in medical devices:
- Wound dressings with colloidal silver or nanosilver
- Silver coatings on endoscopic tubes
- Silver coating of endoprostheses
- Plastics with silver doping for use in medical technology
- Silver-containing creams as medicines and cosmetics, e.g. B. in dandruff with suspected fungal skin or neurodermatitis
- Silver plate as a bone substitute, typically skull bones, for example with Lex Barker after a severe head injury in 1944. In Münchhausen's trip to Russia and St. Petersburg (from 1739) in 1786 there was a story about a hard-drinking general who "picked up a silver plate attached to the same with his hat that served him instead of the skull. "
Examples of hygiene and other uses
- Silver threads or silver ions in the antimicrobial finish of textiles inhibit the growth of bacteria on the skin and thus prevent unpleasant odors.
- Coating of surfaces, e.g. B. in refrigerators, on kitchen furniture, light switches and other objects
- Antibacterial enamels and ceramics
- Silver-coated water filter cartridges
- Coverings of ceramic capacitors for electrical engineering / electronics
With regard to the non-medical use of silver, the Federal Institute for Risk Assessment (BfR) generally recommends for the time being to forego the use of nano-scale silver or nano-scale silver compounds in consumer products.
Silver in catalysis
Silver catalysts are used industrially in the partial oxidation of ethene to ethylene oxide or of methanol to formaldehyde. Due to the importance of silver for oxidation catalysis, numerous studies have been carried out on the interaction of silver surfaces with oxygen. Various oxygen species are localized on the silver surface, in the near-surface area and in the silver volume. In addition to species that are transferred to the substrate and lead more or less selectively to the oxidation of a molecule, there are also centers that enable catalytic dehydrogenation. This is interesting in connection with the fact that the partial oxidation of methanol to formaldehyde requires sub-stoichiometric amounts of oxygen. The formation of the oxygen species depends on the temperature, but also on the type of reaction atmosphere. Certain O species can not be detected ex situ and place high demands on the characterization methods used.
On the other hand, silver also catalyzes the reduction of organic substrates by hydrogen, e.g. B. the hydrogenation of α, β-unsaturated carbonyl compounds. The interaction of H 2 with silver catalysts is - compared to classic hydrogenation catalysts such as platinum - only weak. Ag catalysts are therefore able to selectively hydrogenate double bonds of bi- / multifunctional molecules (e.g. hydrogenation of acrolein to allyl alcohol).
Non-metallic and non-bactericidal silver applications
Silver is also used as a food coloring agent E 174 in the food sector, for example for coatings on confectionery such as pralines and in liqueurs . Silver salts turn glass and enamel yellow.
Silver alloys
Silver can be alloyed with many metals. It can be alloyed well with gold, copper or palladium (a palladium content of 20 to 30 percent makes the silver tarnish-resistant ). To a limited extent, silver can be alloyed with chromium , manganese or nickel. Alloying usually increases the hardness of the silver. It cannot be alloyed with cobalt or iron .
The most important silver alloys today are copper- silver alloys. They are usually designated according to their silver content , expressed in thousandths. The most common silver alloys have a fineness of 800, 835, 925 and 935 parts per thousand of silver. 925 silver is called sterling silver after the British currency pound sterling . It is considered the most important silver alloy and is sometimes used. a. used to make coins, jewelry and cutlery.
With regard to the export today corpus goods primarily made of a silver alloy with a fineness of 935/1000, since the goods with silver solder soldered , be their fineness is lower to ultimately satisfy the legally required total purity of example 925/1000. A new alloy from England is Argentium ™ sterling silver, which should not tarnish . Even with heavily used cutlery, the trend towards sterling silver has been going for years. Silver goods are usually finely silver-plated, while cutlery and wear items are hard silver-plated. The pure silver coating achieves the bright white silver color that promotes sales and greatly reduces the tarnishing of the goods.
A silver alloy used in the Middle Ages to decorate works of art is tula silver , an alloy of silver, copper, lead and sulfur. Silver is also often gilded ; it is then called "Vermeil" with a word derived from French or Latin.
links
Silver occurs in chemical compounds mainly in the oxidation state + I before the oxidation states + II + III + IV and are rare and usually only in complexes stable.
Oxides
- Silver (I) oxide Ag 2 O is a dark brown solid that is obtained from silver-containing solutions with bases , e.g. B. caustic soda fails. At higher temperatures , Ag 2 O breaks down into the elements .
The silver oxides with silver in oxidation states greater than + I can only be produced in an electrochemical way. These are the compounds silver (I, III) oxide AgO, silver (II, III) oxide Ag 3 O 4 and silver (III) oxide Ag 2 O 3 .
Halides
The silver halides are among the most important silver compounds. They decompose in light and are therefore used in analog photography . Silver halides are except the fluoride difficult in soluble water and are used for the detection of halide - ion .
- Silver (I) fluoride AgF is colorless and the only silver halide that is readily soluble in water. In contrast to the other silver halides, it is not photosensitive .
- Silver (I) chloride AgCl is a white, crystalline, water-insoluble powder. It serves as evidence for chloride ions . It is also used in reference electrodes and in analog photography .
- Silver (I) bromide is light yellow and also insoluble in water. Since it is more light-sensitive than silver chloride, it is very often used as a light-sensitive layer in analog photography.
- Like silver bromide, silver (I) iodide is yellow and insoluble in water. It is rarely used in analog photography as well. Silver iodide is sometimes sprayed from airplanes as a condensation nucleus to form rain.
- Silver (II) fluoride AgF 2 is one of the few non-complex divalent silver salts . It is used as a fluorinating agent in place of elemental fluorine .
More connections
- Silver (I) sulfide Ag 2 S is the most difficult of all silver salts to be soluble in water . It is black and is produced directly from the elements or by adding silver containing solution with hydrogen sulfide . When silver cutlery tarnishes, the dark coating also consists of silver sulfide.
- Silver nitrate AgNO 3 is the most important silver compound and raw material for the production of most other silver compounds. It is easily soluble in water and is made by dissolving silver in nitric acid.
- Silver sulfate Ag 2 SO 4 is formed when silver is dissolved in concentrated sulfuric acid .
- Silver azide AgN 3 and silver acetylide Ag 2 C 2 are highly explosive. The former can serve as the initiator of explosives . The also very explosive silver fulminate AgCNO is also known as crack silver.
- Silver cyanide AgCN is a highly toxic, colorless powder that precipitates when cyanide ions are added to silver salt solutions.
Silver in higher oxidation states occurs, for example, in tetrapyridino silver (II) persulfate - [Ag (C 5 H 5 N) 4 ] S 2 O 8 , in potassium tetrafluoroargentate (III) K [AgF 4 ] or cesium hexafluoroargentate (IV) Cs 2 [AgF 6 ] on. The poisonous silver cyanides are u. a. Used in galvanic baths for silver plating and color gold plating (light yellow-greenish-yellow). In the case of silver (I), the tendency to form complex ions is pronounced, usually with the coordination number 2. With the exception of [AgCl 2 ] , which is only formed in strongly hydrochloric acid solution, these complex ions are only stable in alkaline or neutral solution .
proof
When halide solution is added dropwise to the liquid to be tested, precipitates form if silver cations are present, e.g. B .:
- Ag + (aq) + Cl - (aq) → AgCl (s)
As a detection reaction for silver salts, hydrochloric acid or sodium chloride solution is added. A white precipitate of silver chloride forms, which is soluble in dilute ammonia water , forming the silver diammine complex [Ag (NH 3 ) 2 ] + . At high concentrations of chloride, the silver chloride partially dissolves again, as complex dichloroargentates (I) are formed:
- AgCl + Cl - → [AgCl 2 ] -
The precipitate is yellow-greenish with iodide ions ( AgI ) and insoluble in ammonia water, with chloride and bromide ions ( AgCl , AgBr ) whitish.
heraldry
In heraldry , silver, like gold , is referred to as a metal that is one of the heraldic tinctures. It is often represented by white paint.
See also
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1452-1466.
- Günter Ludwig, Günter Wermusch : Silver. From the history of a precious metal. Verlag Die Wirtschaft, Berlin 1986, ISBN 3-349-00101-7 .
Web links
- Mineral Atlas - silver (pictures, deposits, technical facts) , mineral portrait silver
Individual evidence
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1433.
- ↑ The values for the properties (info box) are made of silver , unless otherwise stated . taken from : webelements.com .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e entry on silver in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on silver at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1509.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics. Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b c d e Entry on silver in the GESTIS substance database of the IFA , accessed on April 13, 2020(JavaScript required) .
- ↑ Data sheet Silver, powder, 5-8 μm from Sigma-Aldrich , accessed on June 25, 2020 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 7440-22-4 or silver ), accessed on November 25, 2019.
- ↑ thermal conductivity. on the website of the Technical Faculty of the University of Kiel.
- ↑ a b See silver in the German dictionary of the Brothers Grimm
- ↑ See Online Etymology Dictionary on English silver .
- ↑ Cf. Online Etymology Dictionary on English argent (for silver as heraldic color ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1452-1466.
- ^ IMA / CNMNC List of Mineral Names; July 2019 (PDF 1.67 MB; silver see p. 178)
- ^ IMA / CNMNC List of Mineral Names; 2009 (PDF 1.8 MB, silver see p. 260).
- ↑ Webmineral - Minerals Arranged by the New Dana classification. 01/01/01 Gold group .
- ↑ Mindat - Silver (English).
- ^ Mineral Species Containing Silver (Ag). on: webmineral.com .
- ^ Mindat - Kongsbergite .
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties . 6th, completely reworked and supplemented edition. Weise, Munich 2014, ISBN 978-3-921656-80-8 .
- ↑ Mindat - Coastal Elite .
- ↑ Mineralienatlas : Anthropogenic silver curls
- ↑ S. Jahn: Curly silver from Imiter - real or a fake? In: Min. World . Issue 6, 2008, pp. 28–31.
- ↑ United States Geological Survey: Silver Statistics and Information
- ↑ a b c United States Geological Survey: World Mine Production and Reserves January 2019
- ↑ Trends in the supply and demand situation for mineral raw materials. (PDF; 2.1 MB), Rheinisch-Westfälisches Institut für Wirtschaftsforschung (RWI Essen), Fraunhofer Institute for Systems and Innovation Research (ISI), Federal Institute for Geosciences and Natural Resources (BGR).
- ↑ Jörg Mildenberger: Anton Trutmanns Pharmacopoeia Part II: Dictionary, Volume V. Würzburg 1997, ISBN 3-8260-1398-0 , p. 2274.
- ^ History of plastics - Alexander Parkes. on: plasticker.de
- ^ Inorganic experimental lecture: Silber p. 9, Electrolytic fine cleaning (Möbius process). ( MS Word ; 1.1 MB).
- ↑ Ludwig Hartmann: Faraday to Liebig (1858): To the history of the silver mirror production. In: Sudhoff's archive. 32, 1939/40, pp. 397-398.
- ↑ Silver-coated tube reduces the risk of pneumonia. In: aerzteblatt.de . August 20, 2008, archived from the original on December 26, 2014 ; Retrieved December 26, 2014 .
- ↑ JR Morones-Ramirez, JA Winkler et al: Silver enhances antibiotic activity against gram-negative bacteria. In: Science Translational Medicine . Volume 5, number 190, June 2013, p. 190ra81, doi: 10.1126 / scitranslmed.3006276 . PMID 23785037 .
- ↑ M. Glehr, A. Leithner, J. Friesenbichler, W. Goessler, A. Avian, D. Andreou, W. Maurer-Ertl, R. Windhager, P.-U. Tunn: Argyria following the use of silver-coated megaprostheses. In: The Bone and Joint Journal . Volume 95-B, Issue 7, July 2013, pp. 988-992.
- ↑ Silver (CASRN 7440-22-4). on the website of the American Environmental Protection Agency (EPA).
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Silver , accessed on May 20, 2019.
- ^ Robert Jackson: Witchcraft and the Occult. Quintet Publishing, Devizes 1995, p. 25.
- ↑ Steffen Uttich: Rule change in the middle of the game. In: FAZ.net . July 5, 2008, accessed December 26, 2014 .
- ↑ Overview of the daily development at the record rate, April to May 2011 .
- ↑ Inflation Calculator .
- ^ Michael Höfling: Silver rally in the slipstream of gold. In: welt.de . October 14, 2009, accessed December 26, 2014 .
- ↑ Lex Barker's unofficial fan page - biography (accessed January 31, 2012).
- ↑ BfR advises against using nanosilver in foods and everyday products , statement from 2009 (PDF file, 84 kB), accessed on February 14, 2012.
- ↑ PA Kilty, WMH Sachtler: The Mechanism of the Selective Oxidation of Ethylene to Ethylene Oxide. In: Catalysis Reviews . 10, 1974, pp. 1-16; doi: 10.1080 / 01614947408079624
- ^ H. Sperber: Production of formaldehyde from methanol in BASF. In: Chemical Engineer Technology . 41, 1969, pp. 962-966; doi: 10.1002 / cite.330411705 .
- ↑ A. Nagy, G. Mestl, T. Rühle, G. Weinberg, R. Schlögl: The Dynamic Restructuring of Electrolytic Silver during the formaldehyde synthesis reaction. In: Journal of Catalysis . 179, 1998, pp. 548-559; doi: 10.1006 / jcat.1998.2240 .
- ↑ VI Bukhtiyarov, AI Nizovskii, H. Bluhm, M. Hävecker, E. Kleimenov, A. Knop Gericke, R. Schlögl: Combined in situ XPS and PTRMS study of ethylene epoxidation over silver. In: Journal of Catalysis. 238, 2006, pp. 260-269; doi: 10.1016 / j.jcat.2005.11.043 .
- ↑ A. Knop-Gericke, E. Kleimenov, M. Hävecker, R. Blume, D. Teschner, S. Zafeiratos, R. Schlögl, VI Bukhtiyarov, VV Kaichev, IP Prosvirin, AI Nizovskii, H. Bluhm, A. Barinov , P. Dudin, M. Kiskinova: Chapter 4 X-Ray Photoelectron Spectroscopy for Investigation of Heterogeneous Catalytic Processes. In: Advances in Catalysis . 52, 2009, pp. 213-272; doi: 10.1016 / S0360-0564 (08) 00004-7 .
- ↑ J. Hohmeyer: Characterization of silver catalysts for selective hydrogenation using DRIFT spectroscopy, adsorption calorimetry and TAP reactor. Dissertation . Fritz Haber Institute Berlin / Technical University Darmstadt, 2009.
- ↑ Entry on E 174: Silver in the European database on food additives, accessed on June 16, 2020.





![{\ mathrm {2 \ Ag + H_ {2} O + \ ^ {1} / _ {2} \ O_ {2} +4 \ NaCN \ rightarrow \ 2 \ Na [Ag (CN) _ {2}] \ + \ 2 \ NaOH}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fba93cd5116a374b26aa38f72742866453ea44dc)
![{\ mathrm {Ag_ {2} S \ +4 \ NaCN \ rightarrow \ 2 \ Na [Ag (CN) _ {2}] \ + \ Na_ {2} S}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a6bf0f1c4f727b94f22faf612ed38e2cb4885b6f)
![{\ mathrm {AgCl \ +2 \ NaCN \ rightarrow \ Na [Ag (CN) _ {2}] \ + \ NaCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2a590c9281279d9c39aa718d4ce1a2e9eef95360)
![{\ mathrm {2 \ Na [Ag (CN) _ {2}] \ + Zn \ rightarrow \ Na_ {2} [Zn (CN) _ {4}] + \ 2 \ Ag}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd1b344603946cf96dec47c9df68b469b2610fa0)