promethium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Promethium, Pm, 61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Lanthanoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | La , 6 , f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-12-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-121-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.292 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 1.5 · 10 −15 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | ( 147 μm) 146.9151 u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 185 (205) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 199 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 4 f 5 6 s 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 5.58187 (4) eV ≈ 538.1 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 10.938 (20) eV ≈ 1 055.4 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 22nd.44 (8) eV ≈ 2 170 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 41.17 (9) eV ≈ 3 970 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 61.7 (3) eV ≈ 5 950 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 7.2 g / cm 3 (25 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1353 K (1080 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 3273 K (3000 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 20.10 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 290 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.7 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 1.33 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 15 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.423 V (Pm 3+ + 3 e - → Pm) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Promethium (from Prometheus , a titan of Greek mythology ) is a chemical element with the element symbol Pm and the ordinal number 61. In the periodic table it is in the group of lanthanoids and is therefore also one of the rare earth metals . Promethium was discovered in 1945 as a fission product of uranium. His discovery closed the last gap in the periodic table.
All promethium isotopes are radioactive , which means that all atomic nuclei that contain 61 protons are unstable and decay. Promethium and the lighter technetium (43) are the only elements with a smaller atomic number than bismuth (83) that have this property.
Discovery story
Alleged Discoveries
For the first time a discovery was reported by the Italian scientists Luigi Rolla and Lorenzo Fernandes from Florence. After separating a didymium nitrate concentrate by fractional crystallization from the Brazilian mineral monazite , which consists of 70% dysprosium and neodymium and 30% of the other lanthanoids, they received a solution that mainly contained samarium. This solution resulted in X-ray spectra which they interpreted as samarium and element 61. They named element 61 in honor of their city, Florentium . The results were published in 1926, but the scientists claimed that the experiments were carried out in 1924.
In the same year 1926, Smith Hopkins and Len Yntema at the University of Illinois at Urbana-Champaign also published the discovery of element 61. They named it after the University of Illinois .
Neither of the two publications could be confirmed. Several groups claimed to have created the element, but verification of their discoveries failed due to the difficulty in separating promethium from the other elements.
Evidence by Marinsky, Glendenin and Coryell
Promethium was discovered in 1945 in the Oak Ridge National Laboratory (ORNL) ( Tennessee , USA ) by Jacob A. Marinsky , Lawrence E. Glendenin and Charles D. Coryell as a fission product of uranium . Due to military research during World War II, its discovery was not published until 1947. They chose the name Promethium based on the Greek titan Prometheus, who brought fire to humans and thus aroused the wrath of the gods. This was intended as a warning to humankind, who at that point were beginning the nuclear arms race . The name was suggested by Grace Mary Coryell, Charles Coryell's wife.
Occurrence
Earthly occurrence
In nature, promethium is mostly found as a product of the spontaneous fission of uranium and through alpha decay of the europium isotope 151 Eu. It is found in traces in pitchblende in a concentration of (4 ± 1) · 10 −15 grams 147 μm per kg. The even occurrence of promethium in the earth's crust is about 560 g due to uranium fission and about 12 g due to alpha decay of 151 Eu.
- Spontaneous fission of uranium:
- Alpha decay of 151 Eu:
Alien occurrence
Promethium was detected in the spectrum of the star HR 465 (GY Andromedae) in 1971 ; and possibly in HD 101065 (Przybylski's star) and HD 965 .
Extraction and presentation
In 1963, ion exchange methods were used in the ORNL to obtain approximately 10 grams of promethium from nuclear reactor fuel waste. In 1963 Fritz Weigel was able to produce metallic promethium for the first time by heating promethium (III) fluoride (PmF 3 ) with lithium in a tantalum crucible.
properties
In the periodic table , the promethium with the atomic number 61 is in the series of the lanthanoids, its predecessor is neodymium , the following element is the samarium . Its analogue in the actinoid series is the neptunium .
Physical Properties
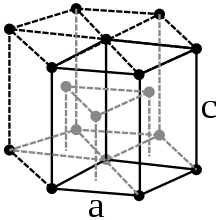
Since the isotope 147 μm can be obtained artificially as a fission product in weighable quantities, it is possible to investigate the properties quite well. Promethium is a silvery white ductile heavy metal. It has a melting point of 1080 ° C; for the boiling point there are estimated values of 2727 and 3000 ° C. Under standard conditions, promethium crystallizes in a hexagonal closest packing of spheres with the lattice parameters a = 365 pm and c = 1165 pm with a calculated density of 7.26 g / cm 3 .
Chemical properties
The metal is oxidized very quickly in air and reacts slowly with water. Promethium occurs in its compounds only in the +3 oxidation state ([Xe] 4f 4 ). It releases the two 6s electrons and one 4f electron. The solutions are purple-tinged pink in color. Among other things, it forms a sparingly soluble fluoride , oxalate and carbonate .
Isotopes
The most stable isotope is 145 μm with a half-life of 17.7 years, followed by 146 μm with a half-life of 5.53 years and 147 μm with a half-life of 2.6234 years. The latter is mostly used for investigation, as it is produced in sufficient quantities as a cleavage product .
use
Due to the short-lived isotopes and the very low availability, this element is only used in small quantities for technical purposes. The most important application is as a beta emitter.
Promethium is used in beta voltaic batteries , the first commercial use of which was to operate a cardiac pacemaker . They are also used as an energy source in satellites in space travel .
The element is a possible mobile source of X-rays that is used for radiometric thickness measurement.
The nuclide 147 μm served not only as a beta radiation source but also as an additive for luminous paint , which was used in luminous numerals of watches and the target optics of weapons such as the M72 (LAW) .
links
→ Category: Promethium compound
Oxides
Promethium (III) oxide (Pm 2 O 3 ) has three different modifications: a hexagonal A-shape (violet-brown), a monoclinic B-shape (violet-pink) and a cubic C-shape (coral red). The melting point is 2130 ° C.
Halides
All halides from fluorine to iodine are known to have an oxidation state of +3.
Promethium (III) fluoride (PmF 3 ) is sparingly soluble in water; it is obtained from a Pm 3+ nitric acid solution by adding HF solution; the precipitate has a pale pink color. Crystalline anhydrous promethium (III) fluoride is a purple-pink salt with a melting point of 1338 ° C.
Promethium (III) chloride (PmCl 3 ) is purple and has a melting point of 655 ° C. If PmCl 3 is heated in the presence of H 2 O, the pale pink colored promethium (III) oxychloride (PmOCl) is obtained.
Promethium (III) bromide (PmBr 3 ) is produced from Pm 2 O 3 by heating in a dry HBr stream. It's red and has a melting point of 660 ° C.
Promethium (III) iodide (PmI 3 ) cannot be prepared from Pm 2 O 3 by reaction with HI - H 2 mixtures; instead, promethium (III) oxyiodide (PmOI) is formed. The desired product is formed by reaction of Pm 2 O 3 with molten aluminum iodide (AlI 3 ) at 500 ° C. It's red and has a melting point of 695 ° C.
More connections
Promethium (III) hydroxide (Pm (OH) 3 ) is obtained from a hydrochloric acid Pm 3+ solution by introducing NH 3 . Its color is purple pink.
safety instructions
Classifications according to the CLP regulation are not available because they only include chemical hazard and play a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
literature
- Fritz Weigel: Chemistry of Promethium , in: Fortschr. Chem. Forsch. , 1969 , 12 (4), pp. 539-621 ( doi: 10.1007 / BFb0051097 ).
-
Gmelin's Handbook of Inorganic Chemistry , System No. 39:
- Part B 1, pp. 1-16, 119, 144-145, 158, 184
- Part B 2, pp. 46, 94-96, 149, 215
- Part B 3, pp. 69, 74-75
- Part B 5, pp. 131-145
- Part B 6, pp. 131-133, 156, 160
- Part B 7, p. 193
- Part C 1, pp. 312-313
- Part C 2, pp. 56-57, 261
- Part C 3, pp. 194, 257
- Part C 4 b, pp. 181-183
- Part C 5, p. 31
- Part C 6, pp. 61-62, 192
- David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-28.
- Comprehensive Inorganic Chemistry , The Lanthanides, pp. 42-44.
- James E. Huheey: Inorganische Chemie , 1st edition, de Gruyter, Berlin 1988, ISBN 3-11-008163-6 , pp. 873-900.
- John Emsley: Nature's building blocks: an AZ guide to the elements , Oxford University Press, 2001, ISBN 0-19-850340-7 , pp. 343-346 ( limited preview in Google book search).
- Eric Scerri : A tale of seven elements , Oxford University Press, Oxford, 2013
Web links
- Entry on Promethium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
Individual evidence
- ↑ a b c d Harry H. Binder: Lexicon of chemical elements , S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 , pp. 487-491.
- ↑ The values for the properties (info box) are taken from www.webelements.com (Promethium) , unless otherwise stated .
- ↑ a b c d e entry on promethium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on promethium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ^ NN Greenwood and A. Earnshaw: Chemistry of the elements , 1st edition, VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1579.
- ↑ a b Weigel: Chemie des Promethiums , p. 577.
- ↑ a b Weigel: Chemie des Promethiums , p. 578.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ Luigi Rolla, Lorenzo Fernandes: About the element of atomic number 61 , in: Journal for inorganic and general chemistry , 1926 , 157 (1), pp. 371–381 ( doi: 10.1002 / zaac.19261570129 ).
- ↑ Luigi Rolla: Florentium or Illinois? , in: Nature , 1927 , 119 , pp. 637-638 ( doi: 10.1038 / 119637a0 ).
- ^ WA Noyes: Florentium or Illinois? , in: Nature , 1927 , 120 , p. 14 ( doi: 10.1038 / 120014c0 ).
- ↑ Luigi Rolla: About the element of the atomic number 61 (Florentium) , in: Journal for inorganic and general chemistry , 1927 , 160 , pp. 190-192 ( doi: 10.1002 / zaac.19271600119 ).
- ↑ Luigi Rolla: Florentium , in: Journal for inorganic and general chemistry , 1927 , 163 , pp. 40-42 ( doi: 10.1002 / zaac.19271630104 ).
- ↑ Luigi Rolla: Florentium. II , in: Journal for inorganic and general chemistry , 1928 , 169 , pp. 319-320 ( doi: 10.1002 / zaac.19281690128 ).
- ^ JA Harris: The Element of Atomic Number 61; Illinois , in: Nature , 1926 , 117 , pp. 792-793 ( doi: 10.1038 / 117792a0 ).
- ↑ Bohuslav Brauner: The New Element of Atomic Number 61: Illinois , in: Nature , 1926 , 118 , pp. 84-85 ( doi: 10.1038 / 118084b0 ).
- ^ RJ Meyer: About the element 61 (Illinois) , in: Naturwissenschaften , 1926 , 14 , pp. 771-772 ( doi: 10.1007 / BF01490264 ).
- ↑ Jacob A. Marinsky, Lawrence E. Glendenin, Charles D. Coryell: The Chemical Identification of Radioisotopes of Neodymium and of Element 61 , in: J. Am. Chem. Soc. , 1947 , 69 (11), pp. 2781-2785 ( doi: 10.1021 / ja01203a059 ).
- ↑ Oak Ridge National Laboratory : Discovery of Promethium ( Memento of the original from July 6, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , in: ORNL Review , 2003 , 36 (1), accessed September 17, 2006.
- ↑ Moses Attrep, Jr., PK Kuroda: Promethium in Pitchblende , in: Journal of Inorganic and Nuclear Chemistry , 1968 , 30 (3), pp. 699–703 ( doi: 10.1016 / 0022-1902 (68) 80427-0 ) .
- ↑ P. Belli, R. Bernabei, F. Cappella, R. Cerulli, CJ Dai, FA Danevich, A. d'Angelo, A. Incicchitti, VV Kobychev, SS Nagorny, S. Nisi, F. Nozzoli, D. Prosperi , VI Tretyak, SS Yurchenko: Search for α Decay of Natural Europium , in: Nuclear Physics A , 2007 , 789 , pp. 15-29 ( doi: 10.1016 / j.nuclphysa.2007.03.001 ).
- ↑ Anonymous : Michigan astronomers discover promethium in star , in: Eos Trans. AGU , 1971 , 52 (1), p. 10 ( abstract ; doi: 10.1029 / EO052i001p00010-01 ).
- ^ MF Aller: Promethium in the star HR 465 , in: Sky & Telescope , 1971 , 41 , pp. 220-222.
- ↑ DN Davis: The Possible Identification of Promethium in S Stars , in: Astrophysical Journal , 1971 , 167 , pp. 327-330 ( full text ).
- ^ SC Wolff, ND Morrison: Remarks on the Proposed Identification of Promethium in HR 465 , in: Astrophysical Journal , 1972 , 175 , pp. 473-475 ( full text ).
- ^ CR Cowley, MF Aller: Comments on the Identification of Promethium in HR 465 , in: Astrophysical Journal , 1972 , 175 , pp. 477-480 ( full text ).
- ↑ O. Havnes, EPJ van den Heuvel, MF Aller, CR Cowley: Is there promethium in HR 465? , in: Astronomy and Astrophysics , 1972 , 19 , pp. 283-286 ( full text ).
- ↑ R. Mitalas, JM Marlborough: Some tests and consequences of the identification of promethium in HR 465 , in: Astrophysical Journal , 1973 , 181 , pp 475-480 ( full text ).
- ↑ CR Cowley, WP Bidelman, S. Hubrig, G. Mathys, DJ Bord: On the possible presence of promethium in the spectra of HD 101065 (Przybylski's star) and HD 965 , in: Astronomy and Astrophysics , 2004 , 419 , p. 1087-1093 ( doi: 10.1051 / 0004-6361: 20035726 ).
- ^ V. Fivet, P. Quinet, É. Biémont, A. Jorissen, AV Yushchenko, S. Van Eck: Transition probabilities in singly ionized promethium and the identification of Pm II lines in Przybylski's star and HR 465 , in: Monthly Notices of the Royal Astronomical Society , September 2007 , 380 (2 ), Pp. 771-780 ( doi: 10.1111 / j.1365-2966.2007.12118.x ).
- ↑ Chung-Sin Lee: Chemical Study on the Separation and Purification of Promethium-147 , in: Journal of Radioanalytical and Nuclear Chemistry , 1989 , 130 , p. 21 ( doi: 10.1007 / BF02037697 ).
- ^ Ion Exchange Purification of Promethium-147 and its Separation from Americium-241, with Diethylenetriaminepenta-acetic acid as the eluant. (PDF; 4.5 MB) (No longer available online.) Archived from the original on June 29, 2011 ; accessed on January 31, 2011 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Fritz Weigel: Representation of metallic promethium , in: Angewandte Chemie , 1963 , 75 (10), pp. 451–451 ( doi: 10.1002 / anie.19630751009 ).
- ↑ PG Pallmer, TD Chikalla: The crystal structure of promethium , in: Journal of the Less Common Metals , 1971 , 24 (3), pp. 233-236 ( doi: 10.1016 / 0022-5088 (71) 90101-9 ).
- ^ A b Robert E. Krebs: The history and use of our earth's chemical elements: a reference guide. 2nd Edition. Greenwood Publishing Group, 2006, ISBN 978-0-313-33438-2 , p. 286 ( limited preview in Google Book Search).
- ↑ Entry on Promethium. In: Römpp Online . Georg Thieme Verlag, accessed on August 15, 2011.
- ↑ Oak Ridge Associated Universities, "Sight for LAW Rocket Launcher," orau.org, July 5, 2016
- ↑ Weigel: Chemie des Promethiums , pp. 591-594.
- ↑ Gmelin, 39 C 1, pp. 312-313.
- ↑ Gmelin, 39 C 3, p. 194.
- ↑ Weigel: Chemie des Promethiums , pp. 587-588.
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1942.
- ↑ Weigel: Chemie des Promethiums , pp. 588-589.
- ↑ Gmelin, 39 C 5, p. 31.
- ↑ Weigel: Chemie des Promethiums , p. 590.
- ↑ Gmelin, 39 C 6, pp. 61-62.
- ↑ Weigel: Chemie des Promethiums , p. 591.
- ↑ Gmelin, 39 C 6, p. 192.
- ↑ Gmelin, 39 C 2, pp. 56-57.




![{\ mathrm {^ {{147}} _ {{\ 57}} La \ {\ xrightarrow [{{4,015} \ s}] {\ beta ^ {{-}}}} \ _ {{\ 58}} ^ {{147}} Ce \ {\ xrightarrow [{{56.4} \ s}] {\ beta ^ {{-}}}} \ _ {{\ 59}} ^ {{147}} Pr \ { \ xrightarrow [{{13.4} \ min}] {\ beta ^ {{-}}}} \ _ {{\ 60}} ^ {{147}} Nd \ {\ xrightarrow [{{10.98} \ d}] {\ beta ^ {{-}}}} \ _ {{\ 61}} ^ {{147}} Pm \ {\ xrightarrow [{{2,6234} \ a}] {\ beta ^ { -}}} \ _ {{\ 62}} ^ {{147}} Sm \ {\ xrightarrow [{{1,06} \ \ cdot \ {10 ^ {{11}}} \ a}] {\ alpha }} \ _ {{\ 60}} ^ {{143}} Nd}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7920036f85206cffc410e608596e74bbbcd0d531)
![{\ mathrm {^ {{151}} _ {{\ 63}} Eu \ {\ xrightarrow [{{5} \ \ cdot \ {10 ^ {{18}}} \ a}] {\ alpha}} \ _ {{\ 61}} ^ {{147}} Pm \ {\ xrightarrow [{{2.6234} \ a}] {\ beta ^ {-}}} \ _ {{\ 62}} ^ {{147 }} Sm \ {\ xrightarrow [{{1.06} \ \ cdot \ {10 ^ {{11}}} \ a}] {\ alpha}} \ _ {{\ 60}} ^ {{143}} Nd}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1464715b125ca605bf9459afb7e59164075c6fbf)