lithium
| properties | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||
| Name , symbol , atomic number | Lithium, Li, 3 | ||||||||||||||||||
| Element category | Alkali metals | ||||||||||||||||||
| Group , period , block | 1 , 2 , s | ||||||||||||||||||
| Appearance | silvery white / gray | ||||||||||||||||||
| CAS number | 7439-93-2 | ||||||||||||||||||
| EC number | 231-102-5 | ||||||||||||||||||
| ECHA InfoCard | 100.028.274 | ||||||||||||||||||
| Mass fraction of the earth's envelope | 60 ppm 27. Frequency | ||||||||||||||||||
| Atomic | |||||||||||||||||||
| Atomic mass | 6.94 (6.938-6.997) u | ||||||||||||||||||
| Atomic radius (calculated) | 145 (167) pm | ||||||||||||||||||
| Covalent radius | 128 pm | ||||||||||||||||||
| Van der Waals radius | 182 pm | ||||||||||||||||||
| Electron configuration | [ He ] 2 s 1 | ||||||||||||||||||
| 1. Ionization energy | 5.391 714 95 (4) eV ≈ 520.22 kJ / mol | ||||||||||||||||||
| 2. Ionization energy | 75.640 096 4 (13) eV ≈ 7 298.16 kJ / mol | ||||||||||||||||||
| 3. Ionization energy | 122.454 358 1 (8) eV ≈ 11 815.05 kJ / mol | ||||||||||||||||||
| Physically | |||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||
| Modifications | 1 | ||||||||||||||||||
| Crystal structure | body-centered cubic | ||||||||||||||||||
| density | 0.534 g / cm³ (20 ° C ) | ||||||||||||||||||
| Mohs hardness | 0.6 | ||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 1.4 10 −5 ) | ||||||||||||||||||
| Melting point | 453.69 K (180.54 ° C) | ||||||||||||||||||
| boiling point | 1603 K (1330 ° C) | ||||||||||||||||||
| Molar volume | 13.02 · 10 −6 m 3 · mol −1 | ||||||||||||||||||
| Heat of evaporation | 136 kJ / mol | ||||||||||||||||||
| Heat of fusion | 3 kJ mol −1 | ||||||||||||||||||
| Speed of sound | 6000 m s −1 at 293.15 K. | ||||||||||||||||||
| Specific heat capacity | 3482 J kg −1 K −1 | ||||||||||||||||||
| Work function | 2.9 eV | ||||||||||||||||||
| Electric conductivity | 10.6 10 6 A V −1 m −1 | ||||||||||||||||||
| Thermal conductivity | 85 W m −1 K −1 | ||||||||||||||||||
| Chemically | |||||||||||||||||||
| Oxidation states | +1 | ||||||||||||||||||
| Normal potential | −3.04 V | ||||||||||||||||||
| Electronegativity | 0.98 ( Pauling scale ) | ||||||||||||||||||
| Isotopes | |||||||||||||||||||
|
|||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||
| NMR properties | |||||||||||||||||||
|
|||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||
Lithium (derived from ancient Greek λίθος líthos 'stone'; pronunciation [ ˈliːti̯ʊm ] or also [ ˈliːʦi̯ʊm ]) is a chemical element with the symbol Li and the atomic number 3. It is an element of the 1st IUPAC group , the group of alkali metals , and belongs to the second period of the periodic table of the elements. Lithium is a light metal and has the lowest density of solid elements under standard conditions .
Lithium does not occur elemental in nature due to its high reactivity. At room temperature it is only stable for a long time in completely dry air, but it reacts slowly to form lithium nitride . In moist air, a matt gray lithium hydroxide layer quickly forms on the surface . Like all alkali metals, elemental lithium reacts even in contact with skin moisture and thus leads to severe chemical burns and burns. In contrast to the corresponding sodium and potassium compounds, many lithium compounds that form lithium ions in aqueous solution are labeled as harmful.
As a trace element , lithium in the form of its salts is a common component of mineral water . Small amounts of lithium are present in the human organism ; however, the element is not essential and has no known biological function. However, some lithium salts have a medicinal effect and are used in lithium therapy for bipolar affect disorders , mania , depression and cluster headaches (see medicine ).
history

The Swede Johan August Arfwedson is considered to be the discoverer of lithium. In 1817 he discovered the presence of a foreign element in petalite (Li [4] Al [4] [Si 4 O 10 ]) and soon after also in spodumene (LiAl [Si 2 O 6 ]) and lepidolite (K (Li, Al) 3 [(Al, Si) 4 O 10 ] (F, OH) 2 ) when he analyzed mineral finds from the island of Utö in Sweden . His academic teacher Jöns Jakob Berzelius suggested lithion , a derivation of the Greek λίθος líthos 'stone', as a name that, according to the names of the other two alkali metals known at the time, sodium and potassium, indicates the material from which it was extracted, and the Lithium finally prevailed in its Latinized form .
In 1818 it was the German chemist Christian Gottlob Gmelin who noticed that lithium salts give a red flame color . Both scientists failed attempts to isolate this element in the years that followed. This was first achieved by William Thomas Brande and Sir Humphry Davy in 1818 using an electrolytic process from lithium oxide (Li 2 O). Robert Bunsen and Augustus Matthiessen produced large amounts of pure lithium in 1855 by electrolysing lithium chloride (LiCl). In 1917 Wilhelm Schlenk synthesized the first organolithium compounds from organic mercury compounds.
With the first commercial production in 1923, the German began Metallgesellschaft in the Hans-Heinrich Hut in Langelsheim in resin by a melt of lithium and potassium chloride (KCl) electrolysis was.
Until shortly after the Second World War, there were hardly any uses for lithium apart from its use as a lubricant (mineral oil, thickened with lithium stearate ) and in the glass industry ( lithium carbonate or lithium oxide ). That changed when the United States needed tritium , which can be extracted from lithium, to make hydrogen bombs . A broad-based production began, especially in Kings Mountain, North Carolina . Due to the large quantities of lithium required due to the short tritium half-life , a large supply of lithium was accumulated between 1953 and 1963, which was only brought onto the market in 1993 after the end of the Cold War . In addition to mining, cheaper extraction from brine now also became important. Larger amounts of lithium are now used for batteries , for the polymerisation of elastomers , in the construction industry and for the organic synthesis of pharmaceuticals and agrochemicals. Since 2007, primary batteries and accumulators ( secondary batteries ) have been the most important segment.
Occurrence and mining
Occurrence on earth
Lithium has a share of about 0.006% of the earth's crust . It occurs somewhat less frequently than zinc and more frequently than cobalt , tin and lead in the earth's crust. Although lithium is more abundant than lead, for example, its greater distribution makes it difficult to obtain. Lithium is found in drinking water and some foods such as meat, fish, eggs and dairy products. 100 g of meat contain around 100 μg of lithium. Various plants such as tobacco or buttercups absorb lithium compounds from the soil and enrich them. The average proportion of the dry matter of plants is between 0.5 ppm and 3 ppm. The mean concentration in the water of the world's oceans is 180 ppb and in river water only around 3 ppb.
Depletion and reserves
In terms of volume, 35,000 tons of lithium were extracted outside the USA in 2015 and mainly traded as lithium carbonate (Li 2 CO 3 ); the reserves in the existing mines are estimated at around 16 million tons (as of March 2018). The world deposits from continental brines, geothermal brines, from the hectorite mineral, oil field brines and from the igneous rock pegmatite is estimated at 53.8 million tons.
The largest resources are in Argentina (9.8 million tons), Bolivia (9 million tons), Chile (8.4 million tons), China (7 million tons), USA (6.8 million tons) and Australia (5 million tons) as well as Canada and the Congo . In Europe, Portugal (100,000 tons) has the largest deposits. Brazil and Mexico have resources of 180,000 tons each.
| Worldwide production [tons] | 2014 | 2015 | 2016 | 2017 (estimated) | Mine reserves | World deposits |
|---|---|---|---|---|---|---|
|
|
n. v. | n. v. | n. v. | 65 | 9,000,000 | 9,000,000 |
|
|
11,500 | 10,500 | 14,300 | 14,100 | 7,500,000 | 8,400,000 |
|
|
2,300 | 2,000 | 2,300 | 3,000 | 3,200,000 | 7,000,000 |
|
|
13,300 | 14,100 | 14,000 | 18,700 | 2,700,000 | 5,000,000 |
|
|
3,200 | 3,600 | 5,800 | 5,500 | 2,000,000 | 9,800,000 |
|
|
300 | 200 | 400 | 400 | 60,000 | 100,000 |
|
|
160 | 200 | 200 | 200 | 48,000 | 180,000 |
|
|
n. v. | n. v. | n. v. | n. v. | 35,000 | 6,800,000 |
|
|
900 | 900 | 1000 | 1000 | 23,000 | 500,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 1,900,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 1,000,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 1,000,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 1,000,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 180,000 |
|
|
n. v. | n. v. | n. v. | n. v. | n. v. | 50,000 |
| world | 16,000,000 | 53,800,000 |
Primary deposits
Lithium occurs in some minerals in lithium pegmatites . The most important minerals are amblygonite (LiAl [PO 4 ] F), lepidolite (K (Li, Al) 3 [(Al, Si) 4 O 10 ] (F, OH) 2 ), petalite (castor; LiAl [Si 4 O 10 ]) and spodumene (triphane; LiAl [Si 2 O 6 ]). These minerals have a lithium content of up to 9% (in the case of amblygonite). Other, rarer lithium ores are cryolite ionite (Li 3 Na 3 [AlF 6 ] 2 ), which has the highest lithium content of all minerals, triphyline (Li (Fe II , Mn II ) [PO 4 ]) and zinnwaldite (K (Li, Fe, Al) 3 [(Al, Si) 4 O 10 ] (F, OH) 2 ). Lithium Minerals come in many silicate - rocks before, but usually only in low concentrations. There are no large deposits. Since the extraction of lithium from these minerals is associated with great effort, they now play a subordinate role in the extraction of lithium or lithium compounds, but this could change due to the expected high demand. The main extraction sites are the Greenbushes and Mt. Cattlin mines in Western Australia , in whose pegmatite rocks there is a high concentration of lithium and in which lithium is a by-product of tantalum extraction . Also in some other countries like Canada and Russia , until 1998 also in Bassemer City , North Carolina , spodumene is mined for lithium production.
Europe has Li-rich pegmatite fields on the Carinthian Weinebene in the Wolfsberg district , in the Finnish region of Ostrobothnia , in the Ore Mountains and between Spain ( Almendra ) and Portugal ( Guarda district , Boticas ).
The deposits in Austria and Finland are being developed by Global Strategic Metals and Keliber and could start operations from 2021. In Austria on the Koralpe in Lavanttal , test tunnels have shown a much larger occurrence of lithium-containing bedrock, which is estimated at 22 million tons. This makes it one of the first lithium mining projects in Europe and could be operated for 20 years. The occurrence near Zinnwald in the Ore Mountains is being explored by Deutsche Lithium .
Secondary deposits
Lithium salts, especially lithium chloride , are also widely found in brine, mostly salt lakes . The concentration can be up to one percent. In addition to the concentration of lithium, the ratio of magnesium to lithium is important for the quality of the brine. Currently, lithium is mainly used in Chile ( Salar de Atacama , which has 0.16% with the highest known lithium content), Argentina ( Salar de Hombre Muerto ), the United States of America ( Silver Peak , Nevada) and the People's Republic of China ( Chabyêr Caka , Tibet; Lake Taijinaier , Qinghai). The Bolivian salt lake Salar de Uyuni, with an estimated 5.4 million tons of lithium, may have the greatest resources. The state-owned company Yacimientos de Litio Bolivianos has been investing with German and Chinese partners in its industrialization, including the neighboring Salar de Coipasa and Laguna Pastos Grandes , since 2018 . There are other lithium-containing salt lakes that are not yet being used for industrial mining as of April 2019, for example in China, Argentina and Afghanistan . In 2016 it became known that in the Paradox Basin in the US state of Utah high-saline deep groundwater ( brine ) had already been encountered in oil exploration wells in the 1960s , from which, according to analyzes at the time, up to 1700 mg / l of pure lithium could be obtained.
Potassium carbonate (potash), borax , cesium and rubidium are often obtained as by- products in lithium production .
Due to the expected strong demand for lithium for batteries in electric vehicles, some companies are currently investigating the extraction of lithium-containing minerals and brine in various regions of the world including Europe. The extraction of lithium from seawater is also being researched. Approx. 230 billion tons of lithium are dissolved in the world's oceans. In 2018, researchers presented an extraction method in which lithium can be obtained from seawater using solar-powered electrolysis . They cited an advantage over conventional extraction that metallic lithium is obtained directly in the process and therefore the (complex and energy-intensive) further processing can be dispensed with, as is necessary in traditional lithium extraction from ores.
Occurrence outside the earth
After the Big Bang , in addition to hydrogen and helium isotopes, a significant amount of the 7 Li isotope was formed. Most of this is no longer available today, because in stars lithium was fused with hydrogen in the process of the proton-proton reaction II and was thus consumed. In brown dwarfs , however, the mass and temperature are not high enough for hydrogen fusion; their mass does not reach the necessary size of about 75 Jupiter masses . The lithium produced during the Big Bang was only preserved in larger quantities in brown dwarfs. For this reason, lithium is a relatively rare element extraterrestrially , but it can be used to detect brown dwarfs.
The distribution of lithium in different stars is very different, even if the age, mass and metallicity are similar. It is believed that planets have an impact on the lithium content of a star. If a star has no planets, the lithium content is high, while stars like the sun, which are surrounded by planets, only have a low lithium content, which is also known as lithium dip . The cause is believed to be that the tidal forces of planets contribute to a stronger mixing of outer and inner layers in stars, so that more lithium gets into an area that is hot enough to fuse it.
Production process
Lithium is mainly obtained from salt water (groundwater, salt lakes) through evaporation. Extraction from rocks in open pit mining is rare .
From salt water
To extract lithium, salty groundwater is pumped to the surface and passed through a chain of evaporation ponds, in which evaporation in the sun takes place over several months. Once the lithium chloride in the ponds has reached the required concentration, the solution is pumped into a processing plant, where unwanted boron or magnesium is extracted and filtered out. Then it is treated with sodium carbonate . The lithium carbonate which precipitates out is filtered and dried. Excess residual brine is pumped back into the salt lake. In arid regions like Chile, the use of groundwater promotes the drying out of the landscape.
presentation
Lithium carbonate is precipitated from lithium-containing salt solutions by evaporating the water and adding sodium carbonate (soda) . To do this, the brine is first concentrated in the air until the lithium content exceeds 0.5%. By adding sodium carbonate, the sparingly soluble lithium carbonate is precipitated:
- .
To obtain metallic lithium, the lithium carbonate is first reacted with hydrochloric acid. This creates carbon dioxide , which escapes as a gas, and dissolved lithium chloride . This solution is concentrated in a vacuum evaporator until the chloride crystallizes out:
The apparatus and systems for the extraction of lithium chloride must be made of special steels or nickel alloys , since the brine is very corrosive . Metallic lithium is fused salt electrolysis of an at 450-500 ° C melting eutectic mixture of 52 percent by mass of lithium chloride and 48 percent by weight potassium chloride was prepared:
The potassium is not deposited during the electrolysis because it has a lower electrode potential in the chloride melt . However, traces of sodium are also deposited and make the lithium particularly reactive (advantageous in organic chemistry, bad for Li batteries). The liquid lithium collects on the electrolyte surface and can thus be discharged from the electrolysis cell relatively easily. It is also possible to produce lithium by electrolysing lithium chloride in pyridine . This method is particularly suitable on a laboratory scale.
Economic importance and raw material trade
After it is obtained lithium passes as a commodity on the trade to the further-processing industries . In commodity trading , especially on the markets for metals , not a pure lithium is traded, that would be chemically unstable. Instead, stable lithium compounds are traded, i. d. Usually with lithium salts or lithium-based crystal heaps , predominantly lithium carbonate or lithium hydroxide monohydrate . These substances are u. a. traded on the London Metal Exchange . In 2020, a price of USD 8.75 / kg was recorded for lithium carbonate (minimum content 99.5%), and a price of USD 10.25 / kg for lithium hydroxide monohydrate (minimum content 56.5%).
In addition to quoting lithium as a raw material, there are 2010 lithium index funds (ETFs) that can be traded on the stock exchange. These ETFs map the market value of companies that are involved in the lithium value chain. Since 2010 there is a stock performance index of Solactive , which tracks the market capitalization of the largest listed companies involved in exploration and mining of lithium and the production of lithium batteries. The ten largest values in this index include (in descending order of size, as of April 2020): Albemarle , SQM , Tesla , BYD , Samsung , Simplo Technology , LG Chem , Panasonic , GS Yuasa and Enersys . The few lithium ETFs mostly track this index.
properties
Physical Properties
Lithium is a silver-white, soft light metal . It is the lightest of all solid elements at room temperature ( density 0.534 g / cm³). Only solid hydrogen at −260 ° C is even lighter with a density of 0.0763 g / cm³.
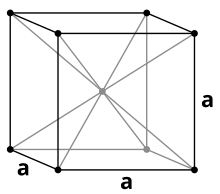
Lithium crystallizes - like the other alkali metals - in a body-centered cubic packing of spheres in the space group Im 3 m (space group no. 229) with the lattice parameter a = 351 pm and two formula units per unit cell . At low temperatures of 78 K, the crystal structure changes through spontaneous transformation into a hexagonal structure of the magnesium type with the lattice parameters a = 311 pm and c = 509 pm or after deformation into a cubic structure of the copper type (cubic face-centered) with the lattice parameter a = 438 pm at. The exact causes of what structure is formed are unknown.
Of the alkali metals, lithium has the highest melting and boiling point and the greatest specific heat capacity . Lithium has the greatest hardness of all alkali metals, but with a Mohs hardness of 0.6 it can still be cut with a knife. As a typical metal, it is a good conductor of electricity (conductivity: about 18% of copper) and heat.
Lithium is largely similar to magnesium , which is also reflected in the fact that heterotypic mixed crystals of lithium and magnesium, known as isodimorphism , occur. Although magnesium crystallizes in the hexagonal closest packing, while lithium crystallizes in the body-centered cubic packing, the two metals are largely heterotypically miscible. However, this only takes place in a limited concentration range, with the component present in excess "forcing" its crystal lattice on the others.
The lithium ion has the highest enthalpy of hydration of all alkali metal ions at −520 kJ / mol . As a result, it is fully hydrated in water and strongly attracts the water molecules. The lithium ion forms two hydration shells, an inner one with four water molecules that are very strongly bonded to the lithium ion via their oxygen atoms, and an outer shell in which other water molecules are connected to the Li [H 2 O] 4 + ion via hydrogen bonds . As a result, the ionic radius of the hydrated ion is very large, even larger than that of the heavy alkali metals rubidium and cesium , which do not have such strongly bound hydration shells in aqueous solution.
As a gas , lithium occurs not only in individual atoms, but also in molecular form as Dilithium Li 2 . The single-bond lithium thereby reaches a full s atomic orbital and thus an energetically favorable situation. Dilithium has a bond length of 267.3 pm and a bond energy of 101 kJ / mol. In the gaseous state, about 1% (by mass) of the lithium is present as dilithium.
Chemical properties
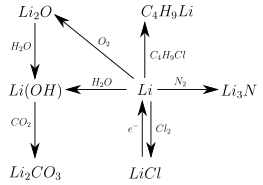
Like all alkali metals, lithium is very reactive and readily reacts with a large number of elements and compounds (such as water ), giving off heat . However, it is the most inert of the alkali metals. A special feature that distinguishes lithium from the other alkali metals is its reaction with molecular nitrogen to form lithium nitride , which takes place slowly even at room temperature:
- .
This is made possible by the high charge density of the Li + ion and thus by the high lattice energy of the lithium nitride. With −3.04 V, lithium has the lowest normal potential in the periodic table and is therefore the least noble of all elements.
Like all alkali metals, lithium is stored under petroleum or paraffin oil, otherwise it will react with the oxygen and nitrogen contained in the air.
Since the ion radii of Li + and Mg 2+ ions are comparably large, there are also similarities in the properties of lithium or lithium compounds and magnesium or magnesium compounds . This similarity in the properties of two elements from neighboring groups in the periodic table is known as an oblique relationship in the periodic table . In contrast to sodium, lithium forms many organometallic compounds ( organolithium compounds ), such as butyllithium or methyllithium . Similar relationships exist between beryllium and aluminum and between boron and silicon .
Isotopes
The two stable isotopes 6 Li (7.6%) and 7 Li (92.4%) occur in nature . In addition, unstable isotopes are known, starting at 4 Li through 8 Li to 12 Li, which can only be produced artificially. Their half-lives are all in the millisecond range .
6 Li plays an important role in nuclear fusion technology. It serves both in the nuclear fusion reactor and in the hydrogen bomb as the starting material for the production of tritium , which is required for the energy-producing fusion with deuterium . Tritium is created in the blanket of the fusion reactor or in the hydrogen bomb next to helium by bombarding 6 Li with neutrons, which arise during the fusion, after the nuclear reaction
- .
The reaction that is also possible
is less suitable (see blanket ) . The separation can take place, for example, via an isotope exchange of lithium amalgam and a dissolved lithium compound (such as lithium chloride in ethanol) (so-called COLEX process ). Yields of around 50% are achieved.
If there is also 7 Li in a three-stage bomb in addition to 6 Li (as was the case with Castle Bravo, for example ), this reacts with some of the fast neutrons generated during the fusion. This creates neutrons again, as well as helium and additional tritium. Although the 7 Li-neutron reaction initially consumes energy, the end result is an increased release of energy through additional fusions and more nuclear fission in the uranium bomb shell . The explosive power is therefore higher than if only the 6 Li part of the isotope mixture had been converted in the bomb. Since it was assumed prior to the Castle Bravo test that the 7 Li would not react with the neutrons, the bomb was about 2.5 times as powerful as expected.
The lithium isotope 7 Li is produced in small quantities in nuclear power plants through a nuclear reaction of the borisotope 10 B (used as a neutron absorber) with neutrons.
The isotopes 6 Li, 7 Li are both used in experiments with cold quantum gases . This is how the first Bose-Einstein condensate with the ( boson ) isotope 7 Li was created. 6 Li, on the other hand, is a fermion , and in 2003 it was possible to transform molecules of this isotope into a superfluid .
use

The most important and fastest growing application for lithium today is its use in lithium-ion accumulators (often also referred to as rechargeable batteries ). B. in smartphones , laptops , battery tools or electrically operated vehicles such as hybrid cars , electric cars or e-bikes (see diagram on the right). Most of the lithium salts produced are not reduced to metal, but either used directly as lithium carbonate, lithium hydroxide, lithium chloride, lithium bromide or converted to other compounds. The metal is only required in some applications. The most important uses of lithium compounds can be found in the " Connections " section.
metal
Some of the lithium metal produced is used for the extraction of lithium compounds that cannot be made directly from lithium carbonate. These are primarily organic lithium compounds such as butyllithium , lithium-hydrogen compounds such as lithium hydride (LiH) or lithium aluminum hydride and lithium amide .
Lithium is used to remove nitrogen from gases because of its ability to react directly with nitrogen .
Metallic lithium is a very powerful reducing agent ; it reduces many substances that do not react with other reducing agents. It is used in the partial hydrogenation of aromatics ( Birch reduction ). In metallurgy it is used for the desulphurisation , deoxidation and decarburization of metal melts.
Since lithium has a very low normal potential , it can be used as an anode in batteries . These lithium batteries have a high energy density and can generate a particularly high voltage . The non-rechargeable lithium batteries are not to be confused with the rechargeable lithium-ion accumulators, in which lithium metal oxides such as lithium cobalt oxide are connected as the cathode and graphite or other compounds that store lithium ions as the anode.
Nuclear fusion
The tritium required for the operation of nuclear fusion reactors is to be produced in the blanket of the lithium-6 reactor.
Alloy component
Lithium is alloyed with some metals to improve their properties. Small amounts of lithium are often sufficient for this. As an admixture, it improves tensile strength , hardness and elasticity in many materials . An example of a lithium alloy is rail metal , a lead alloy with approximately 0.04% lithium, which is used as a bearing material in railroads. The mechanical properties of magnesium-lithium alloys and aluminum-lithium alloys are also improved by adding lithium. At the same lithium alloys are very light and are therefore much in the air and space technology used.
Research (atomic physics)
In atomic physics , lithium is often used because with 6 Li it is the only alkali metal with a stable fermionic isotope , which is why it is suitable for researching the effects in ultra-cold fermionic quantum gases (see BCS theory ). At the same time, it has a very broad Feshbach resonance , which makes it possible to adjust the scattering length between the atoms as desired, whereby the magnetic fields do not have to be kept particularly precise due to the width of the resonance .
medicine
Lithium was first used in Western medicine as a remedy for gout as early as 1850 . However, it turned out to be ineffective. Other approaches to the medical application of lithium salts, including as a remedy for infectious diseases, were also unsuccessful.
It was not until 1949 that the Australian psychiatrist John Cade (1912–1980) described a possible field of application for lithium salts. He had injected guinea pigs with various chemical compounds, including lithium salts, whereupon they reacted less strongly to external stimuli and became calmer, but not sleepy. In retrospect it turned out that the effect observed in the test animals was due to intoxication. After a self-experiment by Cade, the use of lithium carbonate as a drug for the treatment of manic-depressive patients was examined in a double-blind study at the Psychiatric Hospital in Risskov (Denmark) in 1952–1954. This laid the foundation for lithium therapy .
In this, lithium in the form of salts, such as lithium carbonate , is used against bipolar affect disorders , mania , depression and cluster headaches . The low is therapeutic range should be noted that is between 0.6 mmol / l and 1.1 mmol / l and Spiegelbestimungen makes during therapy so required. Even when the lithium blood level is at the upper limit of the therapeutic range, manageable, reversible side effects can occur in sensitive people. However, if the lithium blood level is well above the therapeutic range - i.e. above 1.1 mmol / l - the risk of significant to severe side effects such as tremor , rigor , nausea, vomiting, cardiac arrhythmias and leukocytosis increases rapidly. Above 3.0 mmol / l there is a danger to life. The reason is that the metabolism of lithium and sodium is similar. Excessive lithium levels can result from sweating or sodium-flushing drugs (natriuretic diuretics ) with falling sodium levels. The body tries to compensate for the loss of sodium by withdrawing sodium from the primary urine in the kidneys and transporting it back into the blood (sodium retention ). In addition to sodium, lithium is also retained, which is normally excreted evenly by the kidneys. The result is an increased lithium level, which requires drug monitoring when taking lithium , in which the lithium level is regularly determined and the dose is adjusted accordingly. Even with the correct dosage, long-term treatment with lithium can lead to water and sodium losses ( diabetes insipidus ), hyperacidity of the blood ( acidosis ) and lithium nephropathy with impaired kidney function .
A study published in the USA in 1990 describes a significant reduction in crime and suicide in regions with high lithium levels in drinking water .
The mode of action of lithium as a psychotropic drug has not yet been adequately researched. The influencing of the inositol metabolism by inhibiting myo-inositol-1 phosphatase ( enzyme class 3.1.3.25) and the inhibition of glycogen synthase kinase-3 (GSK-3) in nerve cells are currently being discussed as possible mechanisms. The antidepressant effect of lithium is probably also based on an increase in serotonergic neurotransmission, i.e. an increased release of serotonin in the synapses , while the antimanic effect is explained by an inhibition of dopaminergic receptors. Another interesting effect of lithium salts on humans and mammals such as rats is the probably related change in the circadian rhythm . This effect has even been demonstrated in plants like the Kalanchoe . Other serotonergic substances such as LSD , mescaline, and psilocybin also show such effects in humans. In animal experiments on fruit flies ( Drosophila melanogaster ), lithium has been used to combat symptoms of Alzheimer's disease - such as forgetfulness.
In 2011, the age researcher Michael Ristow showed a possible connection between the content of lithium in the environment and human life expectancy: in a Japanese population study there was a statistically significant connection between a higher content of the trace element and a higher life expectancy; Furthermore, high lithium concentrations extended the life expectancy of the roundworm and model organism Caenorhabditis elegans .
proof
Lithium compounds show a carmine-red flame color , the characteristic spectral lines are the main lines at 670.776 and 670.791 nm; smaller lines are at 610.3 nm. In addition, lithium can be detected with the help of flame photometry .
A quantitative detection with wet chemical methods is difficult, since most lithium salts are easily soluble. One possibility is through the precipitation of the sparingly soluble lithium phosphate . For this purpose, the sample to be examined is made alkaline with sodium hydroxide solution, for example , and a little disodium hydrogen phosphate Na 2 HPO 4 is added. When heated, a white precipitate forms in the presence of Li + :
Another option is to use the iron periodate reagent .
Hazard warnings
Elemental lithium in the form of metal dust ignites in air even at normal temperature. For this reason, metallic lithium must also be stored in the absence of air, usually in petroleum . At higher temperatures above 190 ° C, predominantly lithium oxide is formed immediately on contact with air. In pure oxygen , lithium ignites from around 100 ° C. In a pure nitrogen atmosphere , lithium only reacts more quickly to lithium nitride at higher temperatures. Lithium can react explosively on contact with substances containing oxygen or halogen.
Since lithium reacts strongly exothermic with common fire extinguishing agents such as water , carbon dioxide , nitrogen or the now banned carbon tetrachloride , fires with inert gases such as e.g. B. argon or other metal fire-fighting agents such as salt (e.g. NaCl) can be extinguished.
Elemental lithium, like all alkali metals , causes damage through burns or alkaline chemical burns on skin contact, because it forms lithium hydroxide with water with strong heat emission; the moisture of the skin is sufficient for this.
links
Lithium is very reactive and forms compounds with most non-metals in which it is always in the + I oxidation state . These are usually built up ionically , but in contrast to compounds of other alkali metals have a high covalent content. This can be seen, among other things, in the fact that many lithium salts - in contrast to the corresponding sodium or potassium salts - are readily soluble in organic solvents such as acetone or ethanol . Covalent organic lithium compounds also exist. Many lithium compounds resemble the corresponding magnesium compounds in their properties due to the similar ionic radii ( oblique relationship in the periodic table ). The following graphic provides an overview of the most important reactions of lithium. The stoichiometry and precise reaction conditions are not considered here:
Hydrogen compounds
Hydrogen forms hydrides with lithium . The simplest lithium-hydrogen compound lithium hydride LiH arises from the elements at 600–700 ° C. It is used as rocket fuel and for the rapid production of hydrogen, for example to inflate life jackets . There are also more complex hydrides such as lithium borohydride LiBH 4 or lithium aluminum hydride LiAlH 4 . The latter is of great importance in organic chemistry as a selective hydrogen donor, for example for the reduction of carbonyl and nitro compounds .
Lithium deuteride (LiD) and lithium tritide (LiT) play an important role in nuclear fusion research . Since pure lithium deuteride reduces the energy of the hydrogen bomb, a mixture of LiD and LiT is used for this. These solid substances are easier to handle than tritium, with its fast effusion rate .
Oxygen compounds
With oxygen, lithium forms both lithium oxide Li 2 O and lithium peroxide Li 2 O 2 .
When lithium reacts with water, lithium hydroxide , a strong base, is formed. Lithium hydroxide is used to make lithium greases that are used as greases for cars. Since lithium hydroxide also binds carbon dioxide, it is used to regenerate the air in submarines.
More lithium compounds
Lithium forms salts of the form LiX with the halides. These are lithium fluoride , lithium chloride , lithium bromide and lithium iodide . Since lithium chloride is very hygroscopic, it is used as a desiccant in addition to being used as a starting material for lithium production. It is used to dry gases, for example natural gas , before it is fed through the pipeline or, in air conditioning systems, to reduce the humidity (up to 2% relative humidity). Lithium chloride is also used to lower melting temperatures, in welding and brazing baths and as welding electrode sheathing for welding aluminum. Lithium fluoride is used as a single crystal in infrared spectroscopy .
The technically most important lithium compound is the sparingly soluble lithium carbonate . It is used to extract most other lithium compounds and is used as a flux in the glass industry and in the manufacture of enamel . It is also used in aluminum production to improve the conductivity and viscosity of the melt.
Lithium soaps are lithium salts of fatty acids . They are mainly used as a thickener in high-quality mineral oil- based lubricating greases and waxes and for the production of pencils .
Other lithium salts are:
- Lithium perchlorate LiClO 4 ,
- Lithium sulfate Li 2 SO 4 ,
- Lithium nitrate LiNO 3 , is used with potassium nitrate in the rubber industry for vulcanization ,
- Lithium nitride Li 3 N, is formed when lithium reacts with nitrogen,
- Lithium niobate LiNbO 3 , is transparent in a wide range of wavelengths and is used in optics and for lasers ,
- Lithium amide LiNH 2 , is a strong base and is formed when lithium reacts with liquid ammonia .
- Lithium stearate C 18 H 35 LiO 2 , is an important additive for oils in order to use them as lubricating greases. These are used in automobiles, roller mills and agricultural machinery. Lithium stearates are very sparingly soluble in water, so the lubricating film is retained when they come into contact with little water. The lubricating greases obtained have excellent temperature stability (> 150 ° C) and remain lubricious down to −20 ° C.
- Lithium acetate C 2 H 3 LiO 2
- Lithium citrate C 6 H 5 Li 3 O 7
- Lithium hexafluorophosphate LiPF 6 is used as a conductive salt in lithium-ion batteries.
- Lithium phosphate Li 3 PO 4 is used as a catalyst for the isomerization of propylene oxide.
- Lithium metaborate LiBO 2 and lithium tetraborate Li 2 B 4 O 7
- Lithium bromide LiBr is a reagent for the production of pharmaceuticals, but it is also used in absorption refrigeration systems.
Organic lithium compounds
In contrast to most other alkali metal organyls, lithium organyls play a considerable role, especially in organic chemistry. Of particular importance are n- butyllithium , tert- butyllithium , methyllithium and phenyllithium , which are also commercially available in the form of their solutions in pentane, hexane, cyclohexane or, if appropriate, diethyl ether. They can be prepared by direct reaction of metallic lithium with alkyl / aryl halides
or by transmetallation, for example, from organyl mercury according to
produce.
With elemental lithium in tetrahydrofuran (THF) instead of magnesium in diethyl ether, Grignard- analogous addition reactions of alkyl halides to carbonyl compounds can usually be carried out with better yield.
Due to its clearly covalent character, the structure of organyl lithium can only rarely be described by a simple Li – C bond. There are usually complex structures built up from dimeric, tetrameric or hexameric units or polymeric structures. Lithium organyls are highly reactive compounds, some of which ignite spontaneously in air. They react explosively with water. Due to their extreme basicity, they also react with solvents whose bonded hydrogen is hardly acidic, such as THF , which severely limits the choice of suitable solvents. Reactions with them are only possible under protective gas and in dried solvents. Therefore, some experience is required in dealing with them and great caution is advised.
Another group of organic lithium derivatives are the lithium amides of the LiNR 2 type , of which lithium diisopropylamide (LDA) and lithium bis (trimethylsilyl) amide ( LiHMDS , see also HMDS ) are used as strong bases without nucleophilic activity.
Lithium organyls are used in many ways, for example as initiators for the anionic polymerization of olefins, as metalating , deprotonating or alkylating agents .
The so-called Gilman cuprates of the R 2 CuLi type are of certain importance .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1259-1270.
- NN Greenwood, A. Earnshaw: Chemistry of the Elements. 1st edition. VCH Verlagsgesellschaft, Weinheim 1988, ISBN 3-527-26169-9 , pp. 83-129.
- M. Binnewies: General and Inorganic Chemistry. Spektrum Akademischer Verlag, Heidelberg 2004, ISBN 3-8274-0208-5 , pp. 334–336.
- Ernst Henglein: Technology of Extraordinary Metals. 1991, ISBN 3-8085-5081-3 .
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- Richard Bauer: Lithium - as it is not in the textbook. In: Chemistry in Our Time . 19, No. 5, 1985, pp. 167-173, doi: 10.1002 / ciuz.19850190505 .
- NJ Birch: Inorganic Pharmacology of Lithium. In: Chem. Rev. 99, No. 9, 1999, pp. 2659-2682, PMID 11749496 .
- Jürgen Deberitz, Gernot Boche: Lithium and its compounds - industrial, medical and scientific importance. In: Chemistry in Our Time . 37, No. 4, 2003, pp. 258-266, doi: 10.1002 / ciuz.200300264 .
- Michael Bauer, Paul Grof, Bruno Muller-Oerlinghausen (Eds.): Lithium in Neuropsychiatry: The Comprehensive Guide. 1st edition. Informa Healthcare, 2006, ISBN 1-84184-515-9 .
Web links
- Summary of alkali metals by wiley-vch (PDF; 2.2 MB)
- Batteries for e-cars: five facts about lithium and cobalt (ZDF)
Individual evidence
- ↑ a b c Harry H. Binder: Lexicon of chemical elements . S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (lithium) , unless otherwise stated .
- ↑ The standard value recommended by IUPAC is given, since the isotopic composition of this element can vary locally, the mass range given in brackets results for the mean atomic weight. See: Michael E. Wieser, Tyler B. Coplen: Atomic weights of the elements 2009 (IUPAC Technical Report). In: Pure and Applied Chemistry . 2010, p. 1, doi: 10.1351 / PAC-REP-10-09-14 .
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ a b c Entry on lithium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c Entry on lithium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 97.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics . Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b c d e f M. Hesse, H. Meier, B. Zeeh: Spectroscopic methods in organic chemistry . Thieme, 2002.
- ↑ a b c d Entry on lithium in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ Entry on lithium in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Wolfgang Pfeiler: quanta, atoms, nuclei, particles. Walter de Gruyter GmbH & Co KG, 2017, ISBN 978-3-11-044571-8 , p. 238.
- ↑ Max Mangold: The pronunciation dictionary (= The Duden in 12 volumes. Volume 6). 6th edition. 2005, ISBN 3-411-04066-1 , p. 514.
- ^ Helmut de Boor , Hugo Moser, Christian Winkler (eds.): Siebs: German pronunciation. Pure and moderate high accents with pronunciation dictionary , de Gruyter, Berlin 1969, p. 334 ( limited preview in Google book search).
- ↑ Duden online gives both pronunciation variants, see lithium (with audio samples from the ARD pronunciation database).
- ↑ N. Figurowski: The discovery of the chemical elements and the origin of their names . Aulis-Verlag Deubner, Cologne 1981, ISBN 3-7614-0561-8 , p. 135.
- ↑ C. Elschenbroich: Organometallchemie. 5th edition. Teubner , Leipzig 2005, p. 16.
- ↑ a b Jessica Elzea Kogel: Industrial minerals & rocks: commodities, markets, and uses. 7th edition. SME, 2006, ISBN 0-87335-233-5 , p. 599 ( Industrial minerals & rocks in the Google book search).
- ↑ United States Geological Survey : Minerals Yearbook 2007: Lithium . (PDF; 75 kB), 2007.
- ^ A b c Hans Breuer: dtv-Atlas Chemie. Volume 1, 9th edition. Deutscher Taschenbuch Verlag (dtv), Munich 2000, ISBN 3-423-03217-0 .
- ↑ a b Lithium - a tension maker on the circulatory course. ( Memento from July 17, 2011 in the Internet Archive ) In: VDI nachrichten . January 7, 2011, p. 3.
- ↑ Onmeda Nutrient Lexicon , as of June 10, 2009.
- ^ Lithium Reserves by Country investingnews.com. Retrieved August 8, 2018
- ↑ a b c d e f lithium. at USGS Mineral Resources, 2018. (PDF; 28 kB)
- ↑ Meridian International Research: The trouble with Lithium 2. (PDF; 756 kB) Martainville, May 2008.
- ^ Lithium supply in Portugal (2017).
- ↑ LITHIUM potentialities IN NORTHERN PORTUGAL (2004).
- ↑ [1]
- ↑ Gold of the future: Carinthian lithium mine about to start. Retrieved July 16, 2020 .
- ↑ Zinnwald Lithium Project
- ↑ Zacharias Zacharakis: Lithium: The mountain awakens . In: The time . Hamburg November 13, 2017 ( zeit.de [accessed December 5, 2017]).
- ↑ Alix Arnold: Grandiose landscape and coveted raw material. The Salar de Tunupa / Uyuni in Bolivia is rich in beauty - and lithium. In: ila. Journal of the Latin America Observatory . 395, Bonn, May 2016, pp. 38–39.
- ^ Stephan Bogner: Prima Diamond Corp. acquires the Green Energy project in Utah with historical lithium contents of 1700 mg / L. Rockstone Research, February 18, 2015 ( PDF )
- ↑ The white hope . In: FAZ . January 27, 2011, p. 19.
- ^ Sixie Yang et al .: Lithium Metal Extraction from Seawater . In: Joule . 2018, doi : 10.1016 / j.joule.2018.07.006 .
- ↑ Where all the lithium went. On: Wissenschaft.de from August 15, 2006. Observation of a distant star system brings the solution to a cosmological riddle.
- ↑ About brown dwarfs .
- ↑ Garik Israelian, Elisa Delgado Mena, Nuno C. Santos, Sergio G. Sousa, Michel Mayor, Stephane Udry, Carolina Domínguez Cerdena, Rafael Rebolo, Sofia Randich: Enhanced lithium depletion in Sun-like stars with orbiting planets. In: Nature . No. 462, 2009, pp. 189-191, doi: 10.1038 / nature08483 .
- ^ Terence Bell: An Overview of Commercial Lithium Production. The Balance, May 11, 2018, accessed December 16, 2018 .
- ↑ zdf.de of September 9, 2018, E-Autos: An only apparently clean business, especially the section “Problem Lithium”, accessed on May 4, 2019.
- ↑ ARD: Can the electric car save the environment?
- ^ Lithium at the LME. lme.com Internet portal (London Metal Exchange online), 2020, website accessed on March 21, 2020.
- ↑ Clyde Smith: Advanced battery technology, lithium and graphite mining ETFs: Global X Lithium & Battery Tech ETF (NYSE: LIT), Amplify Advanced Battery Metals (NYSE: BATT). born2invest.com Internet portal, April 10, 2019 (English)
- ↑ Solactive Global Lithium (SOLLIT). investing.com (UK) Internet portal, website accessed March 22, 2020
- ↑ Solactive Global Lithium Index , factsheet, as of April 2, 2020.
- ↑ a b K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica . 30, 1974, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , pp. 928-931.
- ↑ lithium at webelements.com, physical properties .
- ↑ H. Malissa: The separation of lithium from magnesium in lithium-magnesium alloys. In: Fresenius' Journal of Analytical Chemistry . 171, No. 4, 1959, pp. 281-282, doi: 10.1007 / BF00555410 .
- ↑ M. Binnewies: General and Inorganic Chemistry. Spektrum Verlag, 2006, p. 328.
- ^ Mark J. Winter : Chemical Bonding. Oxford University Press, 1994, ISBN 0-19-855694-2 .
- ↑ M. Binnewies: General and Inorganic Chemistry. Spektrum Verlag, 2006, p. 241.
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C . 41, 2017, p. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ Richard Bauer: Lithium - as it is not in the dictionary. In: Chemistry in Our Time . 19, No. 5, 1985, pp. 167-173, doi: 10.1002 / ciuz.19850190505 .
- ↑ Report on the hydrogen bomb test Castle Bravo (Engl.)
- ↑ Martin Volkmer: Nuclear energy basic knowledge. Inforum, 2006, ISBN 3-926956-44-5 , p. 39 ( PDF ( Memento of June 17, 2012 in the Internet Archive )).
- ↑ CC Bradley, CA Sackett, JJ Tollett, RG Hulet: Evidence of Bose-Einstein Condensation in an Atomic Gas with Attractive Interactions. In: Physical Review Letters . 75, No. 9, 1995, pp. 1687-1690, doi: 10.1103 / PhysRevLett.75.1687 .
- ↑ S. Jochim, M. Bartenstein, A. Altmeyer, G. Hendl, S. Riedl, C. Chin, J. Hecker Denschlag, R. Grimm: Bose-Einstein Condensation of Molecules. In: Science . 302, No. 5653, 2003, pp. 2101-2103, doi: 10.1126 / science.1093280 .
- ↑ Script about batteries of TU Graz ( Memento of the original from January 24th, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 3.4 MB).
- ↑ J. Cade: Lithium salts in the treatment of psychotic excitement. In: Med. J. Australia. 36, 1949, pp. 349-352. PMID 18142718 .
- ↑ T. Bschor: 66 years of modern psychopharmaceutical therapy. In: Neurology . 34, 2015, pp. 710-714.
- ↑ M. Schou: Lithium treatment of manic-depressive illness. Thieme, 2001, ISBN 3-13-593304-0 .
- ↑ Gerhard N. Schrauzer, Krishna P. Shrestha: Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. In: Biological Trace Element Research . May 25, 1990, pp. 105-113, PMID 1699579 .
- ↑ MJ Berridge: Inositol trisphosphate and diacylglycerol as second messengers. In: Biochemical Journal . 220, No. 2, 1984, pp. 345-360, PMC 1153635 (free full text).
- ↑ DH Carney, DL Scott, EA Gordon, EF LaBelle: Phosphoinositides in mitogenesis: neomycin inhibits, thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. In: Cell . 42, No. 2, 1985, pp. 479-488, PMID 2992800 .
- ^ R. Williams, WJ Ryves, EC Dalton, B. Eickholt, G. Shaltiel, G. Agam, AJ Harwood: A molecular cell biology of lithium. In: Biochem. Soc. Trans. 32, 2004, pp. 799-802, doi: 10.1042 / BST0320799 .
- ↑ Psychiatric drug therapy .
- ↑ Brigitte Woggon : treatment with psychotropic drugs. Huber, Bern 1998, pp. 77-84.
- ↑ T. Hafen, F. Wollnik: Effect of lithium carbonate on activity level and circadian period in different strains of rats. In: Pharmacology Biochemistry & Behavior . 49, 1994, pp. 975-983, PMID 7886116 .
- ↑ E. Bünning, I. Moser: Influence of Valinomycin on Circadian Lead Movements of Phaseolus. In: Proc. Natl. Acad. Sci. USA . 69, No. 9, 1972, p. 2733, PMC 427027 (free full text, PDF).
- ↑ W. Engelmann: Lithium slows down the clock Kalanchoe. In: Journal of Nature Research B . 27, 1972, p. 477 ( online ). PMID 4403319 .
- ↑ Understanding Hallucinogens. (No longer available online.) Ruprecht-Karls-Universität Heidelberg, archived from the original on January 17, 2012 ; accessed on June 11, 2016 .
- ↑ Sean MJ McBride et al. a .: Pharmacological and Genetic Reversal of Age-Dependent Cognitive Deficits Attributable to Decreased presenilin Function. In: The Journal of Neuroscience . 30, 28, 2010, pp. 9510-9522, doi: 10.1523 / JNEUROSCI.1017-10.2010 .
- ↑ Kim Zarse, Takeshi Terao, Jing Tian, Noboru Iwata, Nobuyoshi Ishii & Michael Ristow: Low-dose lithium uptake promotes longevity in humans and metazoans. In: Eur J Nutr . 50 (5), 2011, pp. 387-389; doi: 10.1007 / s00394-011-0171-x ; PMID 21301855 ; PMC 3151375 (free full text).
- ↑ Fountain of youth in drinking water , Der Spiegel 10/2011.
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 3: H-L. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , pp. 2386-2387.
- ↑ Periodic Table: Lithium . Uniterra.de.
- ↑ PJ Pearce, DH Richards, NF Scilly: A one-step alternative to the Grignard reaction. In: J. Chem. Soc., Perkin Trans. 1 . 1972, pp. 1655-1660, doi: 10.1039 / P19720001655 .






![{\ displaystyle \ mathrm {Li ^ {+} + \ mathrm {e} ^ {-} \ {\ xrightarrow [{Electrolysis}] {(450-500) \, ^ {\ circ} C}} \ Li}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/467e9c877afa1b2a38b28c0a03a93ff0beb790c8)