Gilman reagent
A Gilman reagent is a lithium - containing organic copper compound of the form R 2 CuLi.
history
During his student days, the later discoverer of Gilman reagents, Henry Gilman , traveled to Europe, where he met Victor Grignard, among others . Gilman was fascinated by the advances in organic chemistry in France through Grignard reagents . He devoted a large part of his scientific work to the study of organometallic reagents. He discovered this type of reagent in 1952 as a professor of organic chemistry at Iowa State University .
Manufacturing
Lithiumdimethylkupfer (CH 3 ) 2 CuLi by addition of copper (I) iodide to methyl lithium in tetrahydrofuran obtained at -78 ° C.
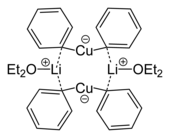
The resulting Gilman reagents have complex structures, both in crystalline form and in solution. In diethyl ether , lithium dimethyl cuprate is present as a dimer in the form of an eight-membered ring, with the lithium atoms coordinating between the methyl groups. Similarly, diphenyl cuprate in solid form forms a dimeric etherate of the form [{Li (OEt 2 )} (CuPh 2 )] 2 . If the lithium ion is complexed with crown ethers , linear diorganyl cuprates are formed.
use
Gilman reagents react with halogenated organic compounds, replacing the halogen with an organic residue. In this way, carboxylic acid chlorides are converted into the ketone in high yield .

Individual evidence
- ^ Henry Gilman, Reuben G. Jones, LA Woods: The Preparation of Methylcopper and some Observations on the Decomposition of Organocopper Compounds. In: The Journal of Organic Chemistry. 17, 1952, pp. 1630-1634, doi : 10.1021 / jo50012a009 .
- ^ Nis Peter Lorenzen, Erwin Weiss: Synthesis and Structure of a Dimeric Lithium Diphenylcuprate: [{Li (OEt) 2} (CuPh2)] 2. In: Angewandte Chemie International Edition in English. 29, 1990, pp. 300-302, doi : 10.1002 / anie.199003001 .
- ↑ Hakon Hope, Marilyn M. Olmstead, Philip P. Power, Janet Sandell, Xiaojie Xu: Isolation and x-ray crystal structures of the mononuclear cuprates [CuMe2] -, [CuPh2] -, and [Cu (Br) CH (SiMe3 ) 2] -. In: Journal of the American Chemical Society. 107, 1985, pp. 4337-4338, doi : 10.1021 / ja00300a047 .
- ↑ JF Normant: Organocopper (I) Compounds and Organocuprates in Synthesis. In: Synthesis. 1972, 1972, pp. 63-80, doi : 10.1055 / s-1972-21833 .
- ^ AE Jukes, SS Dua, H. Gilman: Reactions of some (polyhaloaryl) copper compounds with acid chlorides and chlorosilanes. In: Journal of Organometallic Chemistry. 21, 1970, pp. 241-248, doi : 10.1016 / S0022-328X (00) 90617-X .