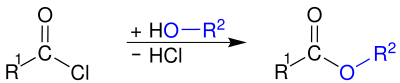Carboxylic acid chlorides
| Carboxylic acid chlorides |
|---|
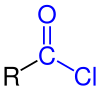
|
| General structure of the carboxylic acid chlorides with the chlorocarbonyl group marked in blue . The radical R represents an aliphatic , cyclic or aromatic radical or a hydrogen atom . |
In chemistry, carboxylic acid chlorides are the most important compounds from the substance group of carboxylic acid halides , which are derived from carboxylic acids. With them, the hydroxyl group of the carboxylic acid has been replaced by a chlorine atom, so that a chlorocarbonyl group results. Depending on the nature of the organic radical, a distinction is made, for example, between alkanoyl chlorides (R = alkyl radical) and aryloyl chlorides (R = aryl radical, e.g. phenyl radical).
nomenclature
Sometimes the terms “carboxylic acid chloride” and “acid chloride” are also used synonymously. This is not entirely correct, because all carboxylic acid chlorides are acid chlorides at the same time. On the other hand, not all acid chlorides are also carboxylic acid chlorides, for example p -toluenesulfonic acid chloride is an acid chloride, but not a carboxylic acid chloride.
It can be named either as carboxylic acid chloride, based on the name of the carboxylic acid, or from the acyl radical . Examples:
- Acetic acid chloride or acetyl chloride , not to be confused with chloroacetic acid , ClCH 2 COOH
- Benzoic acid or benzoyl chloride , not to be confused with ortho -, meta - or para - chlorobenzoic
- Formic acid chloride or formyl chloride is a substance that only exists hypothetically or under high pressure and very low temperatures. Under normal conditions it breaks down into hydrogen chloride and carbon monoxide .
presentation
Carboxylic acid chlorides are synthesized by reacting the corresponding carboxylic acids with thionyl chloride (SOCl 2 ), phosgene (COCl 2 ), phosphorus (III) chloride or phosphorus (V) chloride :
Reaction with thionyl chloride (SOCl 2 ) with elimination of sulfur dioxide and hydrogen chloride :
The reaction with thionyl chloride has the advantage that all of the resulting substances, except for the desired carboxylic acid chloride, are gaseous:
Reaction with phosgene (COCl 2 ) with elimination of carbon dioxide and hydrogen chloride:
Reaction with phosphorus (III) chloride with elimination of phosphonic acid :
Reaction with phosphorus (V) chloride with elimination of phosphorus oxychloride and hydrogen chloride:
The fluorine and bromine analogs react identically.
properties
Most low molecular weight carboxylic acid chlorides are colorless, pungent-smelling liquids that smoke in moist air due to hydrolysis to carboxylic acids and hydrochloric acid . Compared to the respective carboxylic acid, the melting and boiling points are lower because the carboxylic acid chlorides cannot form hydrogen bonds. All carbonic acid chlorides are flammable. Due to the −I effect of the chlorine atom, the carbon atom of the carbonyl group is more positively charged and therefore significantly more reactive than that of the corresponding carboxylic acid.
Reactions
The carboxylic acid chlorides are carbonyl compounds with high reactivity. For this reason, many reactions which, starting from the pure carboxylic acid, are only possible under special conditions, proceed much more easily with the corresponding carboxylic acid chloride.
hydrolysis
Low molecular weight carbonyl chlorides react with water with a stormy, strongly exothermic reaction to form the respective carboxylic acid and hydrogen chloride. The lower the water solubility, the slower the hydrolysis takes place.
For the exact mechanism, see also: addition-elimination mechanism .
Amide formation
The corresponding carboxamides can be produced by reaction with ammonia :
Hydrogen chloride is also split off in the process.
Ester formation
The corresponding carboxylic acid esters can be prepared by reacting with alcohols :
In contrast to that of carboxylic acids and alcohols, this reaction is irreversible.
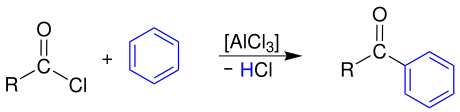
Friedel-Crafts acylation
Aromatic ketones are formed through a reaction with aromatics , here benzene , through Friedel-Crafts acylation :
The carboxylic acid chloride has to be activated beforehand with a Lewis acid, in this case aluminum trichloride , a highly reactive acyl cation which then attacks the aromatic electrophilically .
Formation of carboxylic acid anhydrides
In the laboratory, carboxylic acid anhydrides are produced by the action of carboxylic acid chlorides on alkali salts of carboxylic acids, here a sodium salt.
An alkali chloride, here sodium chloride, is produced as a by-product .
Formation of ketones
A carboxylic acid chloride reacts with an organic cuprate , here lithium dimethyl cuprate , to form a ketone.
Lithium chloride and copper (I) chloride are formed as by-products .
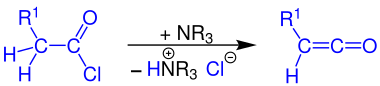
Formation of ketenes
Ketenes can be prepared by reacting a carboxylic acid chloride with a tertiary amine (e.g. triethylamine ) if at least one hydrogen atom is bonded to the α-carbon atom of the acid chloride.
safety instructions
Since the carboxylic acid chlorides hydrolyze very easily and with development of heat, they must be stored as dry as possible. Escaping hydrogen chloride irritates mucous membranes, eyes and skin. The reaction with lower alcohols is usually similarly stormy with the formation of the ester and hydrochloric acid.
Individual evidence
- ^ Hans Beyer and Wolfgang Walter : Organic Chemistry , S. Hirzel Verlag, Stuttgart, 1984, pp. 238-240, ISBN 3-7776-0406-2 .
- ^ A b Hans Beyer and Wolfgang Walter: Organische Chemie , S. Hirzel Verlag, Stuttgart, 1984, p. 241, ISBN 3-7776-0406-2 .
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1236.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 319, ISBN 3-342-00280-8 .
- ^ Hans Beyer and Wolfgang Walter: Organic Chemistry , S. Hirzel Verlag, Stuttgart, 1984, p. 245, ISBN 3-7776-0406-2 .
- ^ Hans Beyer and Wolfgang Walter: Organische Chemie , S. Hirzel Verlag, Stuttgart, 1984, p. 505, ISBN 3-7776-0406-2 .
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , Walter de Gruyter Verlag, Berlin, 4th edition, 2011, p. 553, ISBN 978-3-11-024894-4 .