Benzoyl chloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
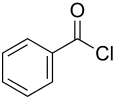
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzoyl chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 5 ClO | ||||||||||||||||||
| Brief description |
colorless, tear-irritating liquid that smokes in moist air and has a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 140.57 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.22 g cm −3 |
||||||||||||||||||
| Melting point |
−1 ° C |
||||||||||||||||||
| boiling point |
197 ° C |
||||||||||||||||||
| Vapor pressure |
50 Pa (20 ° C) |
||||||||||||||||||
| solubility |
Decomposes in water |
||||||||||||||||||
| Refractive index |
1.5537 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
not established, as a suspected carcinogenic effect |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Benzoyl chloride is a chemical compound from the group of carboxylic acid chlorides . It is the acid chloride of benzoic acid .
Extraction and presentation
Benzoyl chloride can be synthesized by reacting benzoic acid with thionyl chloride or phosphorus trichloride .
However, the most important methods of industrial production of benzoyl chloride are the chlorination of benzaldehyde :
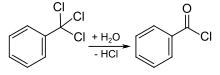
as well as the controlled hydrolysis of benzotrichloride :
or its reaction with benzoic acid:
properties
Benzoyl chloride is a difficultly flammable, colorless liquid (flash point between 55 and 100 ° C), the vapors of which can form an explosive mixture with air if the substance is heated above its flash point. In moist air, the chemical is slightly smoking due to the decomposition to hydrogen chloride and benzoic acid .
Reactions
Benzoyl chloride reacts with water in a hydrolysis reaction to form benzoic acid and hydrochloric acid. Benzamide can be produced by reaction with ammonia , and the corresponding benzoic acid esters are accessible by reacting with alcohols.
use
Benzoyl chloride is u. a. used for the production of benzoic acid esters. Because of its poor solubility in water, the esterification can be carried out according to the Schotten-Baumann method . The use of carboxylic acid chlorides instead of the carboxylic acids gives significantly higher yields.
In the Nucleinsäurenchemie benzoyl chloride used in pyridine as solvent as a popular reagent for introduction of the benzoyl - protecting group to secondary alcohols to protect. The protective group can easily be removed again by alkaline hydrolysis of the benzoic acid ester.
safety instructions
Contact with water (e.g. from the air) produces dangerous hydrogen chloride vapors. Benzoyl chloride causes painful burns on the skin. The unpleasant and pungent smelling vapors irritate the respiratory tract and eyes. In animal experiments, benzoyl chloride (probably because of its extremely rapid metabolism to benzoic acid , hippuric acid and hydrogen chloride) was not found to be genotoxic. Statements on carcinogenicity cannot currently be made.
Individual evidence
- ↑ a b c d e f g h i Entry on benzoyl chloride in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-40.
- ↑ Entry on Benzoyl chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Klaus Schwetlick et al: Organikum . 21st edition. Wiley-VCH, Weinheim 2001, ISBN 3-527-29985-8 , pp. 498 .
- ^ Daniel Krois: Organic-chemical methods. Springer-Verlag, 2016, ISBN 978-3-662-53013-9 , p. 63 ( limited preview in Google book search).
- ↑ BAUA