Secondary (chemistry)
The adjective secondary (abbreviated sec .; From Latin secundarius ) means "in second place" and is also used in chemistry as a word-forming element, e.g. B. in secondary product and secondary metabolite . The short form sec - is used as a descriptor in semi-systematic substance names, for example sec -butanol .
Organic chemistry
|
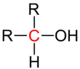
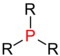
Central atoms marked in red in various groups of substances . Comparison of secondary with primary , tertiary and quaternary central atoms. |
||||
| primary | secondary | tertiary | quaternary | |
|
Carbon atom of an organic compound |

|

|

|

|
| alcohol |

|
|

|
does not exist |
| Amine |

|

|

|
 (see QAV ) |
| Carboxamide |

|

|

|
does not exist |
| Phosphine |

|

|
|
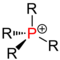 (see QPV ) |
In organic chemistry , the word “secondary” is a term for the degree of substitution of two of several hydrogen atoms bound to a central atom ( carbon , nitrogen , phosphorus ) by organic radicals (organyl radical, such as alkyl radical, alkenyl radical, aryl radical , Alkylaryl radical etc.), e.g. B. secondary alcohols contain a secondary carbon atom (central atom = carbon), secondary amines a secondary nitrogen atom (central atom = nitrogen), secondary phosphines a secondary phosphorus atom (central atom = phosphorus).
Examples of secondary central atoms
- a carbon atom in an alkane or an alcohol which, in addition to two substituents, also bears one or two hydrogen atoms , or
- one nitrogen atom in an amine attached to two carbon atoms.
In the case of secondary compounds, this usually leads to a slight steric hindrance of the functional group and makes typical reactions more difficult than with primary compounds of the same substance class. Examples of such compounds are secondary alcohols , secondary amines and secondary phosphines .
A typical example of a secondary carbon atom is the middle carbon atom in propane or are all six carbon atoms in cyclohexane .
Inorganic chemistry
In inorganic chemistry , the word "secondary" is a name for salts that are formed by neutralizing two hydroxyl groups of a polybasic acid, e.g. B. Calcium hydrogen phosphate, CaHPO 4 .
See also
Individual evidence
- ↑ According to the IUPAC nomenclature, secondary or tertiary amides are substituted with two or three acyl groups on the nitrogen. However, since primary amides with one acyl group and two hydrocarbon substituents on the nitrogen are called "tertiary amides" in common usage, classification into primary, secondary and tertiary amides is not recommended. Entry to amides. In: Römpp Online . Georg Thieme Verlag, accessed on May 15, 2019.
- ^ A b Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1274.
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 5: Pl-S. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1987, ISBN 3-440-04515-3 , p. 3791.