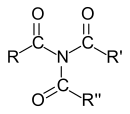
Carboxamides
Carboxamides are derivatives of ammonia and derivatives of primary and secondary amines in which one or more hydrogen atoms on nitrogen have been replaced by acyl groups (R-CO-). As carboxylic acid derivatives, they are also a subgroup of the amides . Carboxylic acid amides are reaction products of carboxylic acid chlorides or carboxylic acid anhydrides with ammonia or amines. Carboxylic acids react with the bases ammonia or amines to form the respective ammonium salts , but not readily to form carboxamides.
nomenclature
In the case of carboxamides, the IUPAC nomenclature differentiates between primary (one carboxy group), secondary (two carboxy groups) and tertiary (three carboxy groups) amides according to the number of carboxy groups on the nitrogen . However, since the designation “primary” or “secondary amide” is unambiguous only for the unsubstituted mono- and diacyl derivatives of ammonia and primary amides with one acyl group and two hydrocarbon substituents on the nitrogen are called “tertiary amides”, the classification becomes not recommended in primary, secondary and tertiary amides.
Diacyl-substituted amides - especially those derived from dicarboxylic acids - are called imides . Amides with one or two alkyl groups on the nitrogen atom are called N- alkylamides or N, N- dialkylamides. Cyclic amides are known as lactams . Peptides and proteins consist of amino acids linked by amide bonds . This type of bond is often called a peptide bond . The polyamide fibers nylon and perlon are technically very important .
presentation
Amides are mainly obtained from the reaction of carboxylic acid derivatives with ammonia or an amine . In the simplest case, the carboxylic acid derivative is a carboxylic acid chloride . The released hydrogen chloride (HCl) reacts with the amine to form a hydrochloride , so that the amine must be used in double the amount:
The aminolysis of carboxylic acid esters yields carboxamides and alcohols:
Carboxylic acids themselves react with amines to form the corresponding salts :
When heated strongly, the salts partially dissociate to form amine and carboxylic acid, which then give the amide with elimination of water:
Other important processes for the synthesis of amides are the Ritter reaction , the hydrolysis of nitriles , the Beckmann rearrangement and the Haller-Bauer cleavage .
properties

The acid amides are mesomeric stabilized . Both the carbonyl carbon and the nitrogen atom are sp 2 - hybridized , all three atoms of the amide group and their neighboring atoms lie in one plane. The CN bond is much shorter (132 pm ) than in other carbon-nitrogen compounds (147 pm ), so that one can assume that a conjugated system is present in which the delocalization of π electrons results in a partial double bond character.
Because of the sp 2 hybridization of nitrogen, its lone pair of electrons is not a protonable n-electron pair, but (as in pyrrole, for example ) a π-electron pair that contributes to conjugation. Amides are therefore extremely weak bases and only form salts with concentrated acids, which are easily hydrolytically split.
The acid amides behave like weak acids. They are deprotonated to amidates by strong bases such as sodium amide . The alkali salts of the amidates are easily decomposed by water. The formation of the amidate ion by deprotonation of the amide is the first step in the Hofmann rearrangement .
The imidic acids are a tautomeric form of the amides.
The reason for the relatively high boiling and melting points of carboxamides compared to carboxylic acids is considered to be their partial zwitterionic character (see figure) and the formation of hydrogen bonds (see figure).

Examples
| Carboxamides | ||
| Surname | structure | Remarks |
| Formamide |  |
The simplest amide, derived from formic acid and ammonia |
| N , N -dimethylformamide (DMF) |  |
Used as an aprotic solvent in organic chemistry and is derived from dimethylamine and formic acid. |
| Acetamide (ethanamide) |  |
Amide of acetic acid |
| urea |  |
A diamide derived from carbon dioxide . |
| ε-caprolactam |  |
A cyclic carboxamide. A raw material for polyamide 6 . |
| Phthalimide |  |
The cyclic imide of phthalic acid . |
| N-benzoylphthalimide | 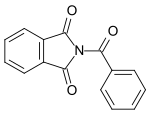 |
Benzoylated phthalimide. |
| For further examples see Category: Amide , Category: Lactam and Category: Imide | ||
Individual evidence
- ↑ Amides and Imides. Rule C-821. In: IUPAC Nomenclature of Organic Chemistry. Advanced Chemistry Development, Inc., accessed May 11, 2019 .
- ↑ a b entry on amides. In: Römpp Online . Georg Thieme Verlag, accessed on February 23, 2019.
- ↑ Amides, imides, and hydrazides. R-5.7.8 (footnote). In: IUPAC Nomenclature of Organic Chemistry, Recommendations 1993. Advanced Chemistry Development, Inc., accessed May 14, 2019 .
- ↑ Hans Beyer, Wolfgang Walter: Textbook of organic chemistry . 18th edition. S. Hirzel Verlag, Stuttgart 1978, ISBN 3-7776-0342-2 , p. 225 .
- ^ Kurt Peter C. Vollhardt, Neil Eric Schore: Organic Chemistry . 5th edition. Wiley-VCH, 2011, ISBN 978-3-527-32933-5 ( limited preview in Google book search).
- ↑ Entry on imidic acids. In: Römpp Online . Georg Thieme Verlag, accessed on February 23, 2019.
- ↑ Joachim Buddrus, Bernd Schmidt: Basics of Organic Chemistry , 5th edition, de Gruyter Verlag, Berlin, 2015, p. 572, ISBN 978-3-11-030559-3 .