Zwitterion
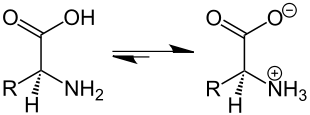
A zwitterion (see also ion ) is a molecule with two or more functional groups , one of which is positively and the other negatively charged. For example, if a zwitterion has two functional groups with opposite charges, the molecule (at the isoelectric point ) is electrically neutral overall. Sometimes the term “inner salt” is also used for a zwitterion.
The groups are usually an acid and a base function . The best-known example are amino acids that are zwitterionic both in aqueous solution and in the solid phase. The acid group releases a hydrogen ion and carries a negative charge, the amino group accepts a hydrogen ion and carries a positive charge. In contrast to betaines , the charges can be compensated by proton migration .
In solution, at a certain pH value , the isoelectric point , as many acid groups are negatively charged as amino groups are positive. Then amino acids no longer migrate in the electric field, but only align themselves, since the total charge is neutral. If the pH is below the isoelectric point, the dissociation of the acid group decreases and the amino acid carries a positive total charge. If the pH is above this, the dissociation of the acid increases and the amino group releases the hydrogen ion, the molecule bears a negative total charge. This effect is used in electrophoresis and isoelectric focusing . The water solubility of amino acids is lowest at the isoelectric point , as the resulting intramolecular charges mean that a stable hydration shell can no longer be formed.
With peptides and proteins , the ratios are analogous to those with amino acids. A proton migrates from a carboxy group of the peptide or protein to a basic amino group, which can be at the N -terminal end, but does not necessarily have to be.
Calculation of the isoelectric point
The pH at the isoelectric point can be (at not too high dilution) from the pK s values of the acid group and the amino group to calculate:
Derivation:
The acid constants are defined as:
Multiplying these two equations eliminates the concentration of the zwitterion:
Since the concentrations of anion and cation are the same at the isoelectric point, the equation simplifies to:
Taking the roots and taking the logarithm then results in:
Multi-proton systems
In acidic (. E.g., aspartic acid , glutamic acid ) or basic amino acids (eg. As lysine , arginine ), the pK for the calculation of the isoelectric point s values of the two carboxyl or amino groups taken into account. To calculate the IEP for amino acids with more than two pK values, only the pK values of the similarly ionizing groups are used. This means that in the case of basic amino acids, the two proximal pK values above the isoelectric point and, in the case of acidic amino acids, the two proximal pK values below the isoelectric point are inserted into the above equation and the mean value is formed to simplify matters, e.g. For lysine (8.95 + 10.53) / 2 = 9.74 and for aspartic acid (2.09 + 3.86) / 2 = 2.98.
Lecithins

Lecithins are phospholipids that are derived from fatty acids , glycerine , phosphoric acid and choline . They are components of the cell membrane of animal and plant life, allow the emulsification (mixing) of fats and water and are therefore important natural surfactants (emulsifiers) for food and feed as well as in the pharmaceutical industry. Two hydroxyl groups in glycerol are esterified with fatty acids. The third OH group in glycerol forms an ester with a phosphate group . The phosphate group is further esterified with the OH group of the choline and thus forms a diester. Choline is a quaternary ammonium compound , so it has a positive charge and is a cation . The phosphate group exists as an anion over a wide pH range , so it has a negative charge. Thus, lecithins can be understood as zwitterions or internal salts .
See also
Individual evidence
- ↑ Entry on zwitterionic compounds / zwitterions . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.Z06752 Version: 2.1.5.
- ↑ Olaf Kühl: Organic Chemistry. Wiley-VCH, Weinheim, 2012, p. 242, ISBN 978-3-527-33199-4 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Verlag Helvetica Chimica Acta, Zurich, 2006, p. 371, ISBN 978-3-906390-29-1 .
- ↑ Reinhard Kuhn: Capillary Electrophoresis: Principles and Practice. Springer Science & Business Media, 2013, ISBN 978-3-642-78058-5 , p. 79.
- ↑ Cherng-ju Kim: Advanced Pharmaceutics. CRC Press, 2004, ISBN 978-0-203-49291-8 , pp. 86-99.
- ↑ WT Godbey: An Introduction to Biotechnology. Elsevier, 2014, ISBN 978-1-908818-48-5 , p. 15.
- ^ Raymond Chang: Physical Chemistry for the Biosciences. University Science Books, 2005, ISBN 978-1-891389-33-7 , p. 291.





