Lysine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
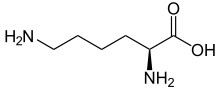
|
|||||||||||||||||||
| Structural formula of the naturally occurring L- lysine | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lysine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 14 N 2 O 2 | ||||||||||||||||||
| Brief description |
colorless needles or hexagonal plates |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 146.19 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lysine , abbreviated to Lys or K , is an essential proteinogenic α - amino acid in its natural L form .
Stereoisomerism
In the proteins, in addition to other amino acids, only L- lysine [synonym: ( S ) -lysine] occurs in peptide bonds . The enantiomer of this is the mirror-image D- lysine [synonym: ( R ) -lysine], which does not occur in proteins . Racemic DL -Lysine [synonyms: ( RS ) -Lysine and (±) -Lysine] is less important than L -Lysine, but has commercial importance as a basic component in drug salts , e.g. B. with acetylsalicylic acid.
Whenever “lysine” is mentioned in this text or in the scientific literature without any additional name ( prefix ), L- lysine is meant.
| Isomers of lysine | ||
| Surname | L- lysine | D -lysine |
| other names | ( S ) -lysine | ( R ) -lysine |
| Structural formula | 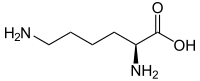 |

|
| CAS number | 56-87-1 | 923-27-3 |
| 70-54-2 ( DL ) | ||
| EC number | 200-294-2 | 213-091-9 |
| 200-740-6 ( DL ) | ||
| ECHA info card | 100,000,268 | 100.011.902 |
| 100,000,673 ( DL ) | ||
| PubChem | 5962 | 57449 |
| 866 ( DL ) | ||
| DrugBank | DB00123 | - |
| - ( DL ) | ||
| Wikidata | Q20816880 | Q27077084 |
| Q178430 ( DL ) | ||
history
After the discovery of phenylalanine , the German chemist Ernst Schulze assumed that the proteins had to be composed of more amino acids than the previously known amino acids. These considerations, among other things, prompted Edmund Drechsel to re- examine the components of the hydrochloric acid breakdown of casein . After treatment with phosphotungstic acid , Drechsel succeeded in isolating the platinum salts of lysine in 1889. The correct composition of this amino acid was published in 1891 by his student Max Siegfried and the final elucidation of the structural formula took place in 1902 through the synthesis of lysine by Nobel Prize winner Emil Fischer and his assistant Fritz Weigert .
properties
Together with L - arginine and L - histidine , L- lysine belongs to the group of basic and at the same time proteinogenic α- amino acids or hexon bases . Lysine has two basic primary amino groups , one in the α-position to the carboxy group and one in the ε-position of the side chain . As with all amino acids, the charge of the lysine is dependent on the pH value. Lysine is mainly present as an "inner salt" or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the ε- amino group , which is more basic than the α-amino group:
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value, here 9.82), at which the lysine also has its lowest solubility in water.
Further physicochemical data for lysine are:
- isoelectric point : 9.74
- Van der Waals volume : 135 Å 3
- Degree of hydrophobicity : −3.9
Industrial manufacture
Several 100,000 tons of L- lysine are produced industrially every year. Today, L- lysine is produced exclusively using the fermentation method, although organic-chemical synthesis routes have been developed.
Occurrence
Lysine is an essential amino acid for humans and other mammals, such as pigs, and must be taken in with the diet . The following examples for the lysine content each relate to 100 g of the food , and the percentage of total protein is also given:
| Food | Total protein | Lysine | proportion of |
|---|---|---|---|
| Beef, raw | 21.26 g | 1797 mg | 8.5% |
| Chicken breast fillet, raw | 23.09 g | 1962 mg | 8.5% |
| Pumpkin seed | 35.49 g | 2283 mg | 6.4% |
| Salmon, raw | 20.42 g | 1870 mg | 9.2% |
| Peas, dried | 24.55 g | 1772 mg | 7.2% |
| Tofu, firm | 15.51 g | 1000 mg | 6.4% |
| Chicken egg | 12.58 g | 914 mg | 7.3% |
| Cow's milk, 3.7% fat | 3.28 g | 260 mg | 7.9% |
| Walnuts | 15.23 g | 424 mg | 2.8% |
| Wholemeal wheat flour | 13.70 g | 378 mg | 2.8% |
| Wholemeal corn flour | 6.93 g | 195 mg | 2.8% |
| Rice, unpeeled | 7.94 g | 303 mg | 3.8% |
| Buckwheat flour | 11.73 g | 595 mg | 5.1% |
| Quinoa | 13 g | 860 mg | 6.6% |
All of these foods contain almost exclusively chemically bound L- lysine as a protein component, but no free L- lysine. Grains usually contain lower levels of L- lysine among the amino acids of the protein component than is optimal for human nutrition.
Estimates of the daily requirement for healthy adults range, depending on the method used, from 8 to 45 mg lysine per kilogram of body weight. In 2002, an expert commission of the FAO / WHO / UNU assumed a daily requirement of between 30 mg and 64 mg per kilogram of body weight for infants and adults.
Functions

Lysine is one of the amino acids that are preferably modified post-translationally . Here, the charge retained (mono- and di- methylation ) or disappear ( acetylation ). In collagen , a modified lysine was found that hydroxylysine having an OH group in the side chain, catalysed , composed of the enzyme lysyl hydroxylase and cofactor ascorbic acid (vitamin C). Hydroxylysine allows the subsequent O - glycosylation of the collagen molecule in the endoplasmic reticulum and Golgi apparatus . Glycosylation determines the packing density of this important connective tissue protein and is also associated with the control of collagen release from the cell ( exocytosis ).
Another modification is ubiquitination in proteins, which are then marked for degradation by the proteasome .
When lysine ( protein rot ) is broken down, the corpse poison cadaverine is produced via pipecolic acid .
use
Most of the industrially produced L- lysine is used in feed supplements to significantly increase the nutritional value of natural feed (grain) with a low L- lysine content .
Racemic DL -Lysine is of commercial importance as a basic component in drug salts, e.g. B. with acetylsalicylic acid (ASA).
L- lysine is a component of infusion solutions for parenteral nutrition and for the treatment of hypochloraemic alkalosis .
Lysine is also used to accelerate the effects of pain relievers, especially in connection with ibuprofen .
biochemistry
For detailed structural formulas see also section Web links
L- lysine can be broken down into two molecules of acetyl-CoA .
further reading
- D. Datta, A. Bhinge, V. Chandran: Lysine: Is it worth more? In: Cytotechnology. Volume 36, number 1-3, July 2001, pp. 3-32, doi : 10.1023 / A: 1014097121364 . PMID 19003311 . PMC 3449675 (free full text).
Web links
Individual evidence
- ↑ a b c d e f Entry on L-lysine. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 698, ISBN 978-0-911910-00-1 .
- ^ A b J. C. Eck and CS Marvel: dl-Lysine Hydrochlorides In: Organic Syntheses . 19, 1939, p. 61, doi : 10.15227 / orgsyn.019.0061 ; Coll. Vol. 2, 1943, p. 374 ( PDF ).
- ↑ a b Lysine data sheet from Sigma-Aldrich , accessed on June 12, 2011 ( PDF ).
- ↑ Sabine Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. ( Memento from June 15, 2016 in the Internet Archive ) Berlin 2015.
- ↑ E. Drechsel, On the Knowledge of the Cleavage Products of Casein. In: Journal for Practical Chemistry. Volume 39, 1889, p. 425ff doi : 10.1002 / prac.18890390135 .
- ↑ M. Siegfried: On the knowledge of the cleavage products of the protein bodies. In: Ber Deutsche Chem Ges. Volume 24, 1891, pp. 418ff
- ↑ E. Fischer, F. Weigert: Synthesis of α, ϵ-Diaminocaproäure (Inactives Lysine). In: Ber Deutsche Chem Ges. Volume 35 (3), 1902, pp. 3772ff
- ↑ Hans-Dieter Jakubke and Hans Jeschkeit. Amino acids, peptides, proteins, Verlag Chemie, 1982, p. 41, ISBN 3-527-25892-2 .
- ↑ PM Hardy: The Protein Amino Acids in GC Barrett (editor): Chemistry and Biochemistry of the Amino Acids , Chapman and Hall, 1985, ISBN 0-412-23410-6 , p. 9.
- ^ Paul G. Higgs, Teresa K. Attwood : Bioinformatics and Molecular Evolution . John Wiley & Sons, 2009, ISBN 1-4443-1118-2 , pp. 24 ( limited preview in Google Book search).
- ↑ a b Y. Izumi et al .: Production and use of amino acids . In: Angewandte Chemie 90, 1978, pp. 187-194. doi : 10.1002 / anie.19780900307
- ↑ nutrient database of the US Department of Agriculture , 21st edition.
- ↑ Firm tofu
- ↑ Jesse P. Greenstein, Milton Winitz: Chemistry of the Amino Acids , Robert E. Krieger Publishing Company, Malabar (Florida), 1961, p. 4, ISBN 0-89874-484-9 .
- ^ GC Barrett: Chemistry and Biochemistry of the Amino Acids , Chapman and Hall, London, New York, 1985, p. 12, ISBN 0-412-23410-6 .
- ^ D. Tomé and C. Bos: Lysine requirement through the human life cycle. 137, 2007, pp. 1642S-1645S PMID 17513440 .
- ↑ Biolys®-the lysine source with added extras
- ^ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag , 1987, p. 406, ISBN 3-13-672201-9 .

