Asparagine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of naturally occurring L -asparagine | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Asparagine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 N 2 O 3 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 132.12 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| solubility |
poor in water (22 g l −1 at 20 ° C, monohydrate, ( L- asparagine)) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Asparagine , abbreviated Asn or N , is one of the proteinogenic α - amino acids in its natural L form .
Asparagine is a derivative of the amino acid aspartic acid , which has an amide group instead of its γ-carboxy group . Therefore - in contrast to aspartic acid - the side chain does not contain an acidic group, but it is polar.
Stereoisomerism
In biosynthetic proteins , only L- asparagine [synonym: ( S ) -asparagine] occurs in peptide bound form alongside other amino acids . The enantiomer of this is the mirror-image D- asparagine [synonym: ( R ) -asparagine], which does not occur in proteins. Racemic DL- asparagine [synonym: ( RS ) -asparagine] is of little importance.
When "asparagine" is mentioned in the literature without any additional name ( descriptor ), it is commonly referred to as L- asparagine .
| Isomers of asparagine | ||
| Surname | L -asparagine | D -Asparagin |
| other names | ( S ) -asparagine | ( R ) -asparagine |
| Structural formula |  |
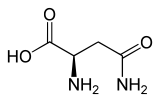
|
| CAS number | 70-47-3 | 2058-58-4 |
| 3130-87-8 ( DL ) | ||
| EC number | 200-735-9 | 218-163-3 |
| 221-521-1 ( DL ) | ||
| ECHA info card | 100,000,669 | 100.016.513 |
| 100.019.565 ( DL ) | ||
| PubChem | 6267 | 439600 |
| 236 ( DL ) | ||
| DrugBank | DB00174 | - |
| - ( DL ) | ||
| Wikidata | Q29519883 | Q27094804 |
| Q185906 ( DL ) | ||
history
L- asparagine is the proteinogenic amino acid that was first discovered. In 1805, the Paris-based professor of chemistry and pharmacy Louis-Nicolas Vauquelin observed the precipitation of two crystals in a residue left by his pupil Pierre-Jean Robiquet after the asparagus juice had been evaporated.
In addition to a sugar-like substance, the two French were able to isolate a salty substance, which they named asparagine, derived from the Latin name asparagus for asparagus . Vauquelin and Robiquet published their more precise results in the following year 1806.
After the isolation and determination of the composition, however, another 57 years passed before the final structure elucidation. It was not until 1862 that Hermann Kolbe succeeded in clearly describing the two structural formulas of asparagine and aspartic acid .
Occurrence
L- asparagine is a component of numerous peptides (e.g. insulin and proteins). Larger natural occurrences are found in asparagus ( Asparagus officinalis ) and in the sprout of butterflies and potatoes.
properties
It has been known since the end of the 19th century that only one of the two stereoisomers ( D- asparagine) tasted sweet, and this was the reason for Louis Pasteur to infer "the chemical asymmetry of nerve substance". L- asparagine, on the other hand, tastes bitter.
Asparagine is mainly present as an "inner salt" or zwitterion , whereby the proton of the carboxy group attaches to the lone pair of electrons on the nitrogen atom of the amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point ( IEP ), which for asparagine has a pH of 5.41. At this pH value, asparagine also has its lowest solubility in water.

Reactions
If a food contains asparagine and reducing sugars (e.g. grape sugar ) at the same time (especially in potatoes and cereals ), acrylamide can be formed at higher temperatures and with a low water content , which has made headlines because of its possible carcinogenic effect.
biochemistry
L -aspartic acid reacts with the aid of asparagine synthetase and the addition of an ammonium ion from glutamine to L -asparagine. Adenosine triphosphate is needed as an energy supplier in this reaction and reacts to adenosine monophosphate and pyrophosphate (PPi). By hydrolysis of L -asparagine turn arises L - aspartic acid .
use
Asparagine is a component of infusion solutions for parenteral nutrition .
See also
Web links
Individual evidence
- ↑ a b c d Data sheet L - (+) - Asparagine, 99% at AlfaAesar, accessed on December 21, 2019 ( PDF )(JavaScript required) .
- ↑ a b c d Entry on L-asparagine. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ S. Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. ( Memento from June 15, 2016 in the Internet Archive ) Berlin 2015.
- ^ PJ Robiquet: Essai Analytique des Asperges. In: Ann. Chim. , Vol. 55 (1), 1805, pp. 152-171 (digitized version ) .
- ↑ L.-N. Vauquelin, P.-J. Robiquet: Découverte d'un Nouvelle Principe Végétal dans le Suc des Asperges. In: Annales de Chimie. Volume 57, 1806, pp. 88–93 (digitized version )
- ↑ H. Kolbe: About the chemical constitution of asparagine and aspartic acid , Liebigs Annalen der Chemie, Volume 121, p. 232ff (1862).
- ↑ Otto-Albrecht Neumüller (editor): Römpps Chemie Lexikon , 8th edition, Frank'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , pp. 293-294.
- ^ Piutti, MA: Sur une nouvelle espèce d'asparagine . In: Comptes rendus . 103, 1886, pp. 134-137.
- ^ Pasteur, L: Observations de M. Pasteur, relatives à la Communication de M. Piutti . In: Comptes rendus . 103, 1886, p. 138.
- ^ Ohloff G: Chemistry of odor stimuli . In: Experientia . 42, No. 3, March 1986, pp. 271-279. PMID 3514264 .
- ^ Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Textbook of food chemistry. 5th edition, Springer Verlag, 2001. ISBN 3-540-41096-1 , p. 33. Restricted preview in the Google book search.
- ^ Gerhard G. Habermehl , Peter E. Hammann and others: Natural substance chemistry. 3rd edition, Springer, 2008, ISBN 3-540-73732-4 , p. 252. Restricted preview in Google book search.
- ^ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag , 1987, p. 66, ISBN 3-13-672201-9 .

