Glutamic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structure of naturally occurring L- glutamic acid | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Glutamic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 9 NO 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 147.13 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.54 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
160 ° C |
|||||||||||||||
| boiling point |
Decomposes at 205 ° C |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Glutamic acid (including α-aminoglutaric acid , 2-aminoglutaric acid ) is an α - amino acid , in the two Spiegelbildisomeren ( enantiomers occurs), one of which proteinogenic form of the human organism itself can produce (not essential amino acid). It is called Glu in the three -letter code and E in the one -letter code . Their salts and esters are called glutamates . In biology and medicine, glutamic acid is usually called glutamate because the compound is dissociated in the body . Glutamic acid is an important building block of proteins ; In addition, glutamate is one of the most important excitatory neurotransmitters in the central nervous system (CNS) of the human organism. As a food additive , L- glutamic acid (E 620) and some of its salts (see glutamates ) are used as flavor enhancers , especially in Asian cuisine and convenience products .
Stereochemistry
Amino acids are chiral molecules. Essentially only L - (+) - glutamic acid [synonym: ( S ) -glutamic acid] occurs in nature . If “glutamic acid” is mentioned in this text or in the scientific literature without any additional name ( prefix ), L- glutamic acid is meant.
The D - (-) - glutamic acid [Synonym: ( R ) -glutamic acid] can be produced by chemical means. They and the racemate from both enantiomers are not discussed in detail in this article.
| Isomers of glutamic acid | ||
| Surname | L -glutamic acid | D -glutamic acid |
| other names | ( S ) -glutamic acid (+) - glutamic acid |
( R ) -Glutamic acid (-) - Glutamic acid |
| Structural formula | 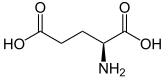 |

|
| CAS number | 56-86-0 | 6893-26-1 |
| 617-65-2 ( DL ) | ||
| EC number | 200-293-7 | 230-000-8 |
| 210-522-2 ( DL ) | ||
| ECHA info card | 100,000,267 | 100.027.273 |
| 100.009.567 ( DL ) | ||
| PubChem | 33032 | 23327 |
| 611 ( DL ) | ||
| DrugBank | DB00142 | |
| - ( DL ) | ||
| Wikidata | Q26995161 | Q27077040 |
| Q181136 ( DL ) | ||
Occurrence
L- glutamic acid occurs in different proportions in most proteins and is present in every protein-containing food . The following examples each relate to 100 g of the food; the percentage of glutamic acid in the total protein is also given. Cheese and meat products are particularly rich in free L -glutamate.
| Food | protein | Glutamic acid | proportion of |
|---|---|---|---|
| Beef , raw | 21.26 g | 3191 mg | 15.0% |
| Chicken breast fillet , raw | 23.09 g | 3458 mg | 15.0% |
| Salmon , raw | 20.42 g | 2830 mg | 13.9% |
| Chicken egg | 12.58 g | 1676 mg | 13.3% |
| Cow's milk , 3.7% fat | 3.28 g | 687 mg | 20.9% |
| Walnuts | 15.23 g | 2816 mg | 18.5% |
| Wholemeal wheat flour | 13.21 g | 4328 mg | 32.8% |
| Wholemeal corn flour | 6.93 g | 1300 mg | 18.8% |
| Rice , unpeeled | 7.94 g | 1618 mg | 20.4% |
| Peas , dried | 24.55 g | 4196 mg | 17.1% |
| Tomato puree | 1.65 g | 658 mg | 39.9% |
history
By digesting gluten with sulfuric acid , the German chemist Heinrich Ritthausen succeeded in isolating glutamic acid for the first time in 1866 at the Agricultural Academy in Waldau near Königsberg. Gustav Werther , professor of chemistry in Königsberg at the time, was able to correctly determine the composition of glutamic acid using the crystals given to him by Ritthausen . After Ritthausen moved to the Agricultural Academy in Bonn-Poppelsdorf, he arranged for the chemist Wilhelm Dittmar to investigate the structure, which Dittmar succeeded in 1872. The results were confirmed by Ludwig Wolff in 1890 .
properties
The isoelectric point of glutamic acid is at pH 3.24. The dicarboxylic acid only slightly dissolves in water (≈11 g / l at 25 ° C) and ethanol ; the solution reacts strongly acidic (pK COOH 2.16, pK γ-COOH 4.32).
Manufacturing
L- glutamic acid is commercially produced exclusively using the fermentation method ( soy sauce , liquid seasoning ). It began with a systematic search for wild-type organisms in which L- glutamic acid could be enriched using suitable nutrient media (starting materials) and culture conditions (temperature, concentration of trace elements, etc.). The fermentation method was optimized by using mutants.
Derivatives
When a mixture of equal parts by weight of glutamic acid and water is heated in an autoclave, pyroglutamic acid , a cyclic amide ( lactam ) , is obtained with elimination of water at reaction temperatures of 135-143 ° C.
Physiological importance
As with all other amino acids, only the L isomer is used as a building block in the metabolism of the human body for glutamate . As a proteinogenic α-amino acid, L- glutamic acid is a component of proteins. In addition, it plays an essential role in cell metabolism in that it is linked to carbohydrate metabolism via the citric acid cycle . In addition, L- glutamic acid is used to form other amino acids.
L -glutamic acid binds the cell toxin ammonia , which is released during protein and amino acid degradation, with the formation of glutamine through the following reaction:
- α-ketoglutarate → glutamic acid → glutamine
L -glutamate is the most important excitatory neurotransmitter in the central nervous system of vertebrates . It is released presynaptically and binds postsynaptically to specific glutamate receptors . In the central nervous system, L-glutamic acid can be decarboxylated to γ-aminobutyric acid (GABA) by the enzyme L-glutamic acid decarboxylase, which acts as a neurotransmitter at inhibitory synapses. L -glutamic acid is the only amino acid that is oxidized, transaminated , aminated and decarboxylated in the brain .
Meaning in the citric acid cycle

L- glutamate is produced in the citric acid cycle from α-ketoglutarate (αKG) and an ammonium ion through the reaction of the enzyme glutamate dehydrogenase (GDH) ( 1 ). Another ammonium ion can be captured by the reaction of glutamine synthetase (GlnS), whereby glutamine is formed ( 3 ). Both reactions serve the spontaneous detoxification of all tissues and are of particular importance in the brain.
For final detoxification, ammonium ions must be added to the urea cycle. This takes place both through transfer ( transamination ) to oxaloacetate (OA) ( 2 ) and via the glutamate dehydrogenase reaction ( 1 ). Glutamine can be converted with α-ketoglutarate in plants into two molecules of L- glutamic acid ( 3 ) and thus supplied to the GDH reaction. This reaction is catalyzed by glutamate synthase (GluS).
The amino acid synthesis is L -glutamic acid of the NH 2 - donor in a transamination. This converts α-keto acids into the homologous α- amino acids . Examples are glutamate oxaloacetate transaminase (GOT) ( 2 ) and glutamate pyruvate transaminase (GPT). Coenzyme is pyridoxal phosphate . Glutamine is the donor for almost all other amino groups that are required in the metabolism.
Glutamic acid (glutamate) in the blood (amino acid concentrations)
The reference ranges (normal values) for glutamic acid in blood findings are in µmol / ml in infants 20–107, in children 18–65 and in adults 28–92. As a therapy for very high levels of glutamic acid (glutamate) in the blood, as it is e.g. B. in the Chinese restaurant syndrome or eczema and / or histamine intolerance , Reinhart Jarisch recommends a vitamin B6 dose in the order of 0.5 mg / kg body weight per day. This also promotes the body's own synthesis of diamine oxidase (DAO) and thus combats the causal effects of histamine intolerance.
Salts
The various salts of glutamic acid are known as food additives . Various salts of glutamic acid with the designation flavor enhancers E 621 to E 625 are used.
Web links
Individual evidence
- ↑ Entry on E 620: Glutamic acid in the European database on food additives, accessed on June 27, 2020.
- ↑ Entry on GLUTAMIC ACID in the CosIng database of the EU Commission, accessed on August 11, 2020.
- ↑ a b c d e f g Entry on glutamic acid in the GESTIS substance database of the IFA , accessed on June 21, 2019(JavaScript required) .
- ↑ a b c d Hans-Dieter Jakubke and Hans Jeschkeit: Amino acids, peptides, proteins , Verlag Chemie, Weinheim, 1982, ISBN 3-527-25892-2 , p. 40.
- ↑ Entry on glutamic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ^ Joint FAO / WHO Expert Committee on Food Additives (JECFA), Monograph for Glutamic acid and its salts , accessed on December 9, 2014.
- ↑ a b ZZulV : Annex 4 (to Section 5, Paragraph 1 and Section 7) Limited additives .
- ↑ nutrient database of the US Department of Agriculture , 21st edition.
- ↑ KH Ritthausen: About the glutamic acid , Journal Prakt Chem, Volume 99 (6-7), p. 454ff (1866).
- ↑ Sabine Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. ( Memento from June 15, 2016 in the Internet Archive ) Berlin 2015.
- ↑ W. Dittmar: About the reduction of glutanic acid by hydrogen iodide , Journal Prakt Chem, Volume 5 (7), p. 308ff (1872).
- ↑ L. Wolff: About glyoxylpropionic acid and some derivatives of the same. Liebigs Annalen der Chemie, Volume 260, pp. 79ff (1890).
- ↑ PM Hardy: The Protein Amino Acids in GC Barrett (editor): Chemistry and Biochemistry of the Amino Acids , Chapman and Hall, 1985, ISBN 0-412-23410-6 , p. 9.
- ↑ Izumi, Y. et al. (1979): Production and Use of Amino Acids . In: Angewandte Chemie 90 (3); 187-194; doi : 10.1002 / anie.19780900307 .
- ↑ Helmut Greiling, AM Gressner: Textbook of clinical chemistry and pathobiochemistry. Schattauer Verlagsgesellschaft, 1987, ISBN 3-79-450949-8 , 1197 pages.
- ↑ Reinhart Jarisch: Histamine intolerance, histamine and seasickness. 2nd edition, Thieme Verlag, Stuttgart New York, 2004, ISBN 3-13-105382-8 , p. 151.
