Structural formula
The term structural formula is a collective term in chemistry for chemical representations that provide information about how atoms in a molecule are connected and arranged in space. Structural formulas show the covalent bonds and - in some cases - the chemical structure . They are used especially in organic chemistry . As structural formulas increasing complexity can electron formulas , valence bond , wedge line formulas and skeletal formula be considered. Projection formulas (e.g. the Fischer projection and the Newman projection ) also occupy a special position .
History of structural formulas
After preliminary work by Charles Frédéric Gerhardt , Hermann Kolbe and Edward Frankland , August von Kekulé was able to specify the number of possible bonds to other atoms for many chemical elements. The number of bonds an atom to other atoms is since 1860 (as suggested by Emil Erlenmeyer ) value called. Kekulé formulated the tetravalence for the carbon atom. In 1865, Kekulé recognized the chemical structure of aromatic compounds such as benzene and naphthalene .
Alexander Butlerow used the name chemical structure at the meeting of naturalists in Speyer in 1861 .
Alexander Crum Brown first used a structural formula (for acetic acid ) in 1864 in which the bonds between the atoms were indicated by dashes.
Overview
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |

|

|
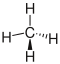
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
construction
In organic chemistry , the so-called skeletal formula is often used, in which C and H atoms are not written out, but implied (assumed). The representation of the carbon structure is done by drawing the bonds between the carbon atoms. A corner is drawn for each carbon atom . Since carbon atoms normally form 4 covalent bonds , the number of hydrogen atoms attached is calculated by subtracting the number of bonds of the carbon atom from 4. The number of delocalized electrons must also be taken into account in aromatic rings . For example, there are 6 delocalized electrons in the benzene ring. As a result, only one hydrogen atom is bonded to each of the six carbon atoms of the ring and not two as in cyclohexane .
example
An example is the representation of hexamethylenetetramine in the skeletal formula :
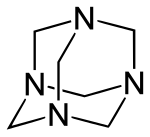
|

As the formula shows, it is common for the skeleton formula to omit the representation of the carbon atoms and the hydrogen atoms . Since a carbon atom can form four bonds and a hydrogen atom can form one bond, the number of hydrogen atoms that are bonded to a carbon atom can be calculated. In the example, further hydrogen atoms are bound to each carbon atom. The name hexamethylenetetramine results from the number of methylene groups (6 = hexa) and the number of amine groups (4 = tetra), cf. Greek numerals .
The structure of benzene and thus also its structural formula was unclear for a long time : The molecular formula of this aromatic hydrocarbon is C 6 H 6 . The corresponding structural formula was constructed in 1865 by Friedrich August Kekulé von Stradonitz . In contrast to the illustration on the stamp on the right, the benzene ring has neither single nor double bonds at fixed positions ( mesomerism ) - all CC bonds in the ring are identical.
Spatial representation
Structural formulas indicate the exact bonding relationships, but sometimes only offer limited information about the exact spatial structure of a molecule. The compressions necessary for the reduction to two dimensions lead to the fact that bond lengths and angles are distorted.
In order to clarify the spatial representation of molecules in a drawing, bonds can be drawn as wedges or dashed lines. A wedge represents a bond that protrudes out of the plane of the paper, while a dashed line represents a bond that goes into the plane of the paper. This is particularly important for the representation of the structure of chiral substances.
A precise spatial representation can be achieved using the dome model. In the past, kits were used for this purpose; today, three-dimensional representation on the computer is common (see molecular modeling ).
Semi-structural formula
Semi-structural formulas are used to supplement and simplify the representation.
It is often not possible to determine which substance is involved from empirical formulas . However, since it is often not necessary or very time-consuming to draw the structural formula of a molecule, semi-structural formulas were introduced.
The empirical formula C 3 H 8 O only indicates the number of atoms in a molecule, but does not provide any information on the substance class of the compound. The semi-structural formulas CH 3 -CH 2 -CH 2 -OH (1), CH 3 -CH (OH) -CH 3 (2) and CH 3 -CH 2 -O-CH 3 (3), which sum up the same Contain number of types of atoms, illustrate this and state that (1) and (2) are alcohols , and (3) is an ether . In this form the semi-structural formula corresponds to the constitutional formula .
In addition to the “real” semi-structural formulas (CH 3 –CH 2 –CH 2 –OH), the common form C 3 H 7 OH, which lies between the empirical formula (C 3 H 8 O) and the semi-structural formula, has become established in everyday laboratory work , although this is often not is unique. In the example C 3 H 7 OH, it remains open whether this is 1-propanol ( n -propanol ) or the isomeric 2-propanol ( iso -propanol ). However, the notation n -C 3 H 7 OH for 1-propanol or iso -C 3 H 7 OH or i -C 3 H 7 OH for 2-propanol is clear . CH 3 - (CH 2 ) 2 -OH or H 3 C- (CH 2 ) 2 -OH are occasionally found as clear intermediate forms for 1-propanol .
See also
- SMILES (chemical structure code )
- Chemical Markup Language (document format for structural formulas)
Individual evidence
- ↑ Entry on structural formula . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S06061 Version: 2.1.5.