Skeletal formula
The skeletal formula (also scaffold formula , English skeletal formula ) an organic compound is a structural formula , a concise representation of the molecular structure allows. Skeletal formulas are ubiquitous in organic chemistry because they can not only clearly show complicated structures, but are also quick and easy to draw.
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |
|
|
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|
|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
The carbon skeleton
The term skeleton refers to the carbon skeleton of an organic compound, which forms the basis of an organic compound through the main chains , side chains and / or rings . Hydrogen atoms are the most common atoms bonded to carbon atoms and, like carbon atoms, are not shown explicitly. All other atoms are called heteroatoms ; they form functional groups . These are also referred to as substituents , as they substitute (Latin: replace) a certain hydrogen atom bound to carbon in the molecule.
Implied carbon and hydrogen atoms

In valence bar formulas , carbon atoms are represented by the element symbol “C”, and hydrogen atoms by “H”. The presence and the position of these atoms are not shown in skeletal formulas, but they are automatically assumed. H. implies. The representation of the carbon structure is done by drawing the bonds between the carbon atoms. A corner is drawn for each carbon atom .
Since carbon atoms normally form 4 atomic bonds, the number of attached hydrogen atoms is calculated by subtracting the number of bonds of the carbon atom from 4. For example, the skeletal formula of hexane is shown on the right . The carbon atom labeled “C 1 ” has only one bond, so three hydrogen atoms must be bonded to this carbon atom. In comparison, “C 3 ” must have attached two hydrogen atoms because it has formed two bonds.

The hydrogen atoms of functional groups , on the other hand, are shown explicitly. An example is the hydroxyl group of ethanol . The functional groups are written as a unit and without connecting lines for the sake of clarity and compactness. In some cases, e.g. B. to highlight their role in certain reaction mechanisms , however, these are shown.
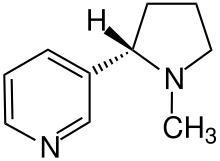
Furthermore, individual hydrogen atoms are also shown if their position is relevant for a stereochemical characterization, such as with nicotine .
Explicit heteroatoms
All atoms that are not carbon or hydrogen, i.e. all heteroatoms , are represented with their element symbol, e.g. B. "Cl" for chlorine , "O" for oxygen or "Na" for sodium .
Pseudo elements
Some characters look like chemical elements , but represent common functional groups or any atom from a group. B. "Ph" used for the phenyl group .
There are also:
Isotopes
elements
- X for any halogen atom
- M for any metal atom
Alkyl groups
- R for any alkyl group or any possible substituent at all
- Me for the methyl group
- Et for the ethyl group
- n -Pr for the propyl group
- i -Pr for the isopropyl group ( iso -propyl group)
- Bu for butyl group , wherein usually the n -butyl group is meant
- i -Bu for the isobutyl group ( iso -Butyl group)
- s -Bu for the sec -Butyl group
- t -Bu for the tert -butyl group
- Pn for the pentyl group , usually meaning the n -pentyl group
- Hx for the hexyl group , mostly referring to the n -hexyl group
- Hp for the heptyl group , usually referring to the n -heptyl group
- Cy for the cyclohexyl group
Aromatic substituents
- Ar for every aromatic substituent - from aryl radical - (Ar is also the symbol for the element argon . Since only one argon compound is known so far, there is no danger of confusion)
- Bn for the benzyl group
- Bz for the benzoyl group
- Ph for the phenyl group
- Tol for the tolyl group , mostly referring to the p -tolyl group (4-methylphenyl group)
- Xy for the xylyl group
Functional groups
- Ac for the acetyl group (Ac is also the symbol for the element actinium . Actinium compounds, however, are so rare that this convention can hardly lead to confusion)
Leaving groups
See the leaving group article for more information
- Bs for the brosyl group
- Ns for the nosyl group
- Tf for the triflyl group
- Ts for the tosyl group
Multiple bonds
Two atoms can be bound by more than one electron pair . Because of the geometric and physical possibilities, there are single , double and triple bonds . Single bonds are represented by simple lines between two atoms, double bonds by two parallel lines, and triple bonds by three parallel lines.
In more complex theories of attachment there are non-integer attachment values. In this case, a combination of solid and dashed lines is drawn to represent the integer and non-integer parts of the bond.
Hex-3-ene has an internal carbon-carbon double bond
1-hexene has a terminal (final) double bond
Hex-3-yne has an internal carbon-carbon triple bond
Hex-1-yne has a terminal (final) triple bond
NB in the illustration above, double bonds are drawn in red and triple bonds in blue. The color marking is only for reasons of clarity. Multiple bonds are usually not shown in color.

It is in the nature of the representation that long-chain alkanes cannot be drawn linearly in the skeletal formula (the carbon atoms are indicated by the kinks between the bonds). The planar representation of tetrahedral angles can be derived from the angles in the carbon chain . As a result, a skeletal formula always correctly represents the cis - trans isomerism that occurs with double bonds.
In the example above, the trans and (E) configurations of the double bonds are shown. The triple bonds are not shown in tetrahedral form, since no further hydrogen atom can be attached to carbon-carbon triple bonds and therefore no cis - trans isomerism can exist. Shown on the right are two cis or (Z) -C = C double bonds, in which the carboxy group COOH has been partially drawn.
Benzene rings
Benzene rings are common in organic compounds. To show the delocalization of the electron pairs over the six carbon atoms in the ring, a circle is drawn within a hexagon made of single bonds. This style is very common, especially in school books.
An alternative to this, which is often used in science, is the Kekulé structure . This representation is considered imprecise, as it assumes three single and three double bonds (benzene would therefore be cyclohexa-1,3,5-triene). However, the advantages of the representation are that with it reaction mechanisms can be clearly represented. However, the use of the Kekulé representation requires knowledge of the electron pair delocalization.
Stereochemistry
Stereochemical properties are appropriately represented in skeletal formulas:
- Solid lines represent atomic bonds in the plane of the representation
- Wedge-shaped lines represent bonds that lie in a higher layer, i.e. in a plane that is closer to the viewer
- Dashed lines represent bonds that lie in a deeper layer, i.e. in a plane that is further away from the viewer
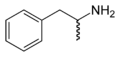
- Curved, wavy lines represent either unknown stereochemistry or a racemic mixture of both possible enantiomers .
The skeletal formula of amphetamine intended to express its racemic nature.
Other ways of representing ties
Hydrogen bonds are sometimes indicated by dotted or dashed lines. In some cases the wedge-shaped lines mentioned under stereochemistry are also used to determine the oxidation number . In this case the wedge points to the more electronegative atom of a bond. The counting is then relatively simple, by counting −I for each wedge and + I for each tip for each atom.
Web links
- Drawing organic molecules , chemguide.co.uk (English)