Nicotine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Natural substance nicotine | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Nicotine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 14 N 2 | |||||||||||||||||||||
| Brief description |
colorless to brownish oily liquid with a tobacco ( pyridine ) like odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Means of smoking cessation |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 162.23 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.01 g cm −3 |
|||||||||||||||||||||
| Melting point |
−79 ° C |
|||||||||||||||||||||
| boiling point |
246 ° C |
|||||||||||||||||||||
| Vapor pressure |
5.6 Pa (20 ° C) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
easily in water , ethanol , diethyl ether and chloroform , miscible with many organic solvents |
|||||||||||||||||||||
| Refractive index |
1.5282 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 0.07 ml m −3 or 0.5 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Nicotine , also known as nicotine , is an alkaloid naturally occurring in the leaves of the tobacco plant and, in lower concentrations, in other nightshade plants , which has stimulating or paralyzing effects on ganglia of the autonomic nervous system . Nicotine derivatives are rarely referred to as nicotinoids ; usually the synthetic neonicotinoids used as insecticides are meant.
history
The tobacco plant was ritually consumed in America by the Maya since the 10th century at the latest. In 1492, Christopher Columbus was presented with dried tobacco on his arrival in the New World . The ambassador of France to Portugal, Jean Nicot de Villemain, sent seeds of Nicotiana tabacum to the French king in 1560 , promoting their medicinal use. Nicotine was first isolated under the name Nicotianine in 1828 by the chemist Karl Ludwig Reimann and the physician Christian Wilhelm Posselt as part of a competition at the University of Heidelberg ; they chose the name after Jean Nicot. The chemical structure was clarified by Adolf Pinner and Richard Wolffenstein . In 1851, the Belgian chemist Jean Servais Stas proved that Hippolyte Visart de Bocarmé had poisoned his victim Gustave Fougnies with nicotine.
Occurrence
Natural occurrence
Nicotine is mainly produced as a secondary metabolite in significant quantities by various species of the genus Nicotiana (especially Nicotiana tabacum and Nicotiana rustica ) and other genera of the nightshade family (for example Duboisia hopwoodii and Asclepias syriaca ) . Nicotine is also found in very low concentrations in some other species of the family and the closely related bindweed family . Outside of these families, the substance occurs sporadically in lower concentrations, for example in the genus Erythroxylum from the redwood family . Nicotine is also found in smaller quantities in various nightshade plants such as potatoes , tomatoes and eggplant . Since nicotine is formed almost exclusively in the roots of the tobacco plant, nicotine-free tobacco can be made from tobacco plants that have been grafted onto the roots of tomato plants .
Nicotine content of tobacco products and substitutes
The nicotine content of the smoke of a cigarette is measured in a standardized smoking machine about 0.6 to 2.4 milligrams , of which 0.9 to 1.2 mg are ingested by the smoker . The nicotine content in dried tobacco is 0.6 to 2.9 percent of the dry matter . It should be noted, however, that the indication of the amount of nicotine per cigarette has only an extremely limited information quality, since the content of nicotine ingested varies depending on the type of inhalation and the construction of the cigarette. Furthermore, it is essential that a smoker does not necessarily consume less nicotine per day by switching to nicotine-reduced cigarettes, as many smokers pull on these more and longer. The cigarette itself contains significantly more nicotine (approx. 12 mg, see section on toxic effects ), which when smoked, however, simply burns for the most part before it is inhaled.
A typical nicotine patch releases about one milligram of nicotine per hour over 16 or 24 hours.
The snuff can be a daily Nicotinaufnahmemenge similar to that of a heavy smoker result (20 to 60 mg).
properties
Constitutive phytochemicals
Nicotiana , the Latin name for the genus of tobacco plants , produce nicotine in their roots. When the plant ripens, the substance migrates into the leaves, where it reaches a mass fraction of 0.5 to 7.5 percent. The nicotine serves in the plant parts, especially in the leaves, to ward off predators of the plant , provided that the predator has a nervous system with nicotinic acetylcholine receptor . Nicotine and nicotinoids are powerful insecticides .
Physical Properties
Pure nicotine is a colorless, oily liquid at room temperature that quickly turns brown when exposed to air. It is a water-soluble base and volatile in water vapor.
| Enantiomers of nicotine | ||
| Surname | ( S ) nicotine | ( R ) nicotine |
| other names |
L- nicotine (-) - nicotine |
D- nicotine (+) - nicotine pseudonicotine |
| Structural formula |  |
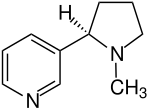
|
| CAS number | 54-11-5 | 25162-00-9 |
| 22083-74-5 (mixture of isomers) | ||
| EC number | 686-240-2 | |
| 623-834-2 (mixture of isomers) | ||
| ECHA info card | 100.211.968 | |
| 100.152.478 (mixture of isomers) | ||
| PubChem | 89594 | 157672 |
| 942 (mixture of isomers) | ||
| Wikidata | Q28086552 | Q27119762 |
| Q12144 (mixture of isomers) | ||
Chemical properties
The chemical structure of nicotine, which is based on two connected rings made of pyridine and pyrrolidine , was elucidated by Adolf Pinner and Richard Wolffenstein . Nicotine has a stereogenic center , it is chiral . Only ( S ) -nicotine occurs in nature . Natural nicotine is in the same configuration as L- proline at the center of chirality . The enantiomer ( R ) -nicotine has no pathophysiological significance. Whenever the term 'nicotine' is used in this article, it always means ( S ) -nicotine.
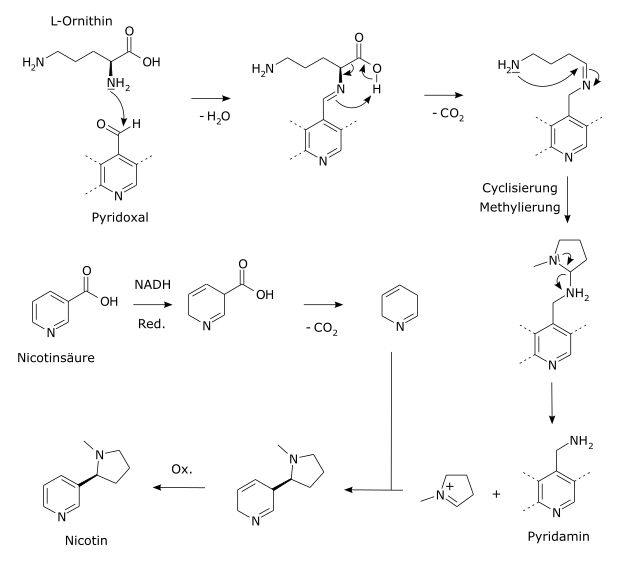
biosynthesis
In tobacco plants, nicotine is synthesized from nicotinic acid and L- ornithine in the following steps:
- 1,4-Reduction of the pyridine ring of nicotinic acid to 1,4-dihydronicotinic acid , using NADPH as a reducing agent.
- Decarboxylation of 1,4-dihydronicotinic acid to 1,2-dihydropyridine .
In parallel:
- Formation of putrescine from L- ornithine.
- Synthesis of an N -methylpyrrolinium cation from putrescine.
Reaction to finished nicotine:
1,4-Dihydronicotinic acid (an enamine) reacts with the N -methylpyrrolinium cation (an iminium ion) via an intermediate and subsequent reoxidation of the dihydropyridine ring with NADP + to give nicotine.
Analytics
The reliable qualitative and quantitative determination of nicotine in the various test materials is possible after appropriate sample preparation by coupling gas chromatography or HPLC with mass spectrometry . The headspace technique is also used in special cases.
Biochemical meaning and effect
The absorption of nicotine takes place differently depending on the pH value. Cigar tobacco is made from leaves that are harvested when they are not fully ripe. As a result, the carbohydrates in the leaf are largely broken down during drying and fermentation, so that mainly basic protein breakdown products are present in the smoke. The smoke from cigars therefore usually has a pH value of 8.0–8.6. In cigarette tobacco, on the other hand, nicotine is partly present in a protonated form in salt-bound form, with a smoke pH range of 6.3 to 5.6. The absorption of free nicotine from alkaline smoke occurs well through the mucous membranes. About 2 - 5% of the nicotine contained in tobacco is absorbed. For the physiological effect of the nicotine from acid cigarette smoke intended by the smoker, however, inhalation is necessary, with about 10-20% of the nicotine contained in the mainstream smoke remaining in the body. It arrives in the brain at a comparatively high influx speed of 10 to 20 seconds after inhalation. There the nicotine has a stimulating effect on the nicotinic acetylcholine receptors. This type of receptor is found in parasympathetic ganglia , sympathetic ganglia, in the adrenal medulla , central nervous system, and on the motor endplates . Nicotine activates parasympathetic nerves and inhibits sympathetic nerves in their activity. Nicotine also promotes the release of the hormone adrenaline and the neurotransmitters dopamine and serotonin . In small amounts, nicotine has a stimulating effect. Nicotine briefly and reversibly accelerates the heartbeat and causes a narrowing of the v. a. the peripheral blood vessels; this leads to an increase in blood pressure , slight sweating (decrease in skin resistance ) and, as a result of the narrowing of the peripheral blood vessels, a decrease in skin temperature. The central effects include, above all, an increase in psychomotor performance as well as attention and memory skills and relaxation in some negative affective states. However, this increase is only during the duration of action. Nicotine intake reduces appetite. There is an increase in gastric juice production due to the release of histamine and increased bowel activity. In addition, nicotine is also known to have an anti- diuretic effect. On the effects of nicotine and the release of dopamine, a will gain in consumer behavior triggered, which can result in nicotine dependence. Withdrawal symptoms such as irritability or dysphoric moods can last up to 72 hours. In healthy cells, nicotine activates protein kinase B , which controls the metabolism , growth and death of cells. This increases the survivability of the cells.
Nicotine is not on the doping list , although it increases stamina.
According to the criteria of a longitudinal study, studies related to the tobacco industry came to the conclusion that nicotine has a beneficial and protective influence on Alzheimer's disease , whereas the other publications do not and identify it as a risk factor . In further studies, however, a positive effect of nicotine in relation to the development and therapy of the disease is documented. The consumption of nicotine is associated with a lower likelihood of developing Parkinson's disease. In albino laboratory rats, a harmful effect on embryos during pregnancy was demonstrated by a nicotine salt (nicotine bi-tartrate), which epigenetically manifested itself as asthma in the next generation and the next but one . It is not known whether such an effect exists in humans. Nicotine and some metabolites are being studied in the treatment of Parkinson's disease and depression in nonsmokers.
Pharmacokinetics and pharmacokinetic interactions
The plasma half-life of nicotine is 1–2 hours. 10% of nicotine is excreted unchanged through the kidneys. The rest is mainly metabolized by CYP2A6 to cotinine, which is partly excreted and partly further metabolized with a significantly longer plasma half-life.
Polycyclic aromatic carbohydrates in cigarette smoke and tobacco tar induce the activity of the cytochrome CYP1A2 and CYP2B6, which accelerates the breakdown of CYP1A2 substrates. CYP1A2 is involved in the oxidative metabolism of a number of drugs and environmental toxins and accelerates their breakdown, so that as a result, therapeutically desired plasma levels of pharmaceuticals cannot be reached or maintained. Among other things, this applies to some psychotropic drugs and antidepressants. Since this effect is not due to nicotine, it is not influenced by nicotine replacement treatment. Nicotine is broken down in the body into cotinine , nicotine- N ' -oxide , nornicotine , hydroxynicotine and being tainted .
Other toxins that act on acetylcholine receptors are anatoxin-a some cyanobacteria , conine the spotted hemlock , arecoline of betel nuts , cytisine of laburnum and Epibatidin of poison dart frog .
Toxic effect
Nicotine is primarily a stimulant in low doses . In a medium dose, on the other hand, it has a relaxing effect. The phenomenon of the dose-dependent change in effect has been described as the Nesbitt paradox . Nicotine is only very toxic to higher animals in high concentrations , since in high doses it blocks the ganglia of the autonomic nervous system . Nicotine is the active ingredient in tobacco that is mainly responsible for the addictive potential of tobacco consumption. Acute overdoses are associated with nausea and vomiting.
In the kidneys, blood pressure rises under the action of nicotine, accompanied by a reduced glomerular filtration rate and reduced local flow of blood plasma. In adolescents, nicotine can lead to changes in the development of the nucleus accumbens , the middle prefrontal cortex , the basolateral amygdala , the bed nucleus of the stria terminalis and the dentate gyrus .
The rate of nicotine absorption through human skin is generally slow and depends on the solvent. The pure base (100% nicotine) is taken up extremely slowly at a rate of 82 µg / cm² per hour; H. if you apply pure nicotine to 10 cm² of skin, you ingest 0.8 mg per hour (which is roughly equivalent to smoking half a cigarette). When applying a 20 percent solution of nicotine in an alcoholic solution to 10 cm², the absorption is 0.1 mg nicotine per hour. In a dilute aqueous solution (20 percent) nicotine uptake is significantly faster at 8.8 mg per hour.
For a long time it was assumed that even if 60 mg of nicotine were swallowed, an adult was in danger of death. This assumption was based on the research results of the toxicologist and pharmacologist Rudolf Kobert. In 1906 he published the Textbook of Intoxications , in which he relied on experimental results of 2 to 4 mg and deduced from them that the maximum lethal oral dose of nicotine could not be higher than 60 mg. Kobert traced his surveys back to self-experiments by the Austrian doctor Karl Damian von Schroff in 1856. In 2014, the pharmacologist Bernd Mayer from the Karl-Franzens University in Graz corrected the value to over 500 mg.
In the case of cigarettes swallowed by children, an American 4-year study with 700 analyzed cases showed that the course of the disease was always easy when up to two cigarettes were swallowed. The Swiss Toxicological Information Center therefore recommends that children only consult a doctor if more than two cigarettes have been swallowed or symptoms of intoxication (such as vomiting, reddened skin, paleness, restlessness) occur. In some cases, however, a medical consultation is seen as mandatory even for smaller quantities.
Information on nicotine and tar content may no longer be given on the packaging of a cigarette packet, as this information has turned out to be misleading. The consumer could come to the assumption that the choice he has made is healthier in comparison. The packs are to be provided with warning notices according to strict specifications. The upper limits for the amount of nicotine supplied through the smoke is limited to 1 milligram, the amount of tar to 10 milligrams and the amount of CO to 10 milligrams per cigarette by EU Directive 2014/40. The tobacco in a cigarette contains on average around 12 milligrams of nicotine.
At the suggestion of the Dutch chemicals authority, the chemical classification of nicotine was reviewed in 2015. The Committee for Risk Assessment (RAC) of the European Chemicals Agency (ECHA) changed the classification for nicotine on September 10, 2015 as follows: The classification is in the acute toxicity category 2, both orally, dermally and by inhalation, the warnings are extended to H300 , H310 and H330 (fatal if swallowed, skin contact and inhalation) and H411 (Aquatic Chronic 2). This classification of the RAC was then implemented by the EU Commission into applicable law, which has to be taken into account by companies and authorities since May 1, 2020.
Carcinogenic effects
Nicotine is not on the list of carcinogenic substances of the International Agency for Research on Cancer of the World Health Organization .
Cancer-promoting effect
The US Journal of Clinical Investigation reported that nicotine, as part of chemotherapy, blocks the body's ability to destroy cells with damaged genetic material. Such cells, however, have to be broken down by the body as quickly as possible during such a therapy, because otherwise the cancerous tumors already in the body will continue to multiply with less hindrance. In healthy cells, nicotine activates protein kinase B. This increases the survivability of the cells, which is beneficial in principle, but harmful if they later mutate into cancer cells. In addition, it has been reported that nicotine promotes the formation of new blood vessels ( angiogenesis ) and thus any existing cancerous tumors are better supplied with nutrients and can grow faster.
Dependency potential
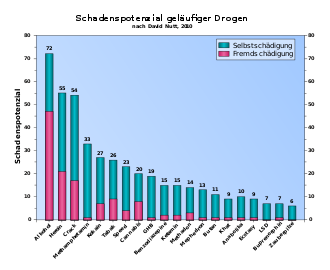

Nicotine is mainly responsible for the dependence on tobacco products . The addictive effect of nicotine is intensified by the monoamine oxidase inhibitors contained in tobacco smoke . Comparisons of animal studies and studies on human drug consumption show that pure nicotine is very addictive, while tobacco cigarette smoke is very addictive. Lewin referred to this fact in Phantastica as early as 1924. Nicotine, in connection with other substances in tobacco smoke, has an extremely high potential for dependence and can very quickly lead to dependent behavior . According to a paper published in 2007 by David Nutt et al. a. the addiction potential of tobacco smoke lies somewhere between alcohol and cocaine. More precisely, the potential for physical dependence is that of alcohol or barbiturates and the potential for psychological dependence is that of cocaine. A comparison with addiction to opiates such as heroin is not indicated because this is much more complicated to treat and the withdrawal symptoms are more severe. A few cigarettes or a few days with small cigarette consumption are enough to become physically dependent. The potential for dependence on orally ingested nicotine is significantly lower, and patches have almost no potential for dependence.
Connection with the use of other substances
In animal experiments it is relatively easy to determine whether the consumption of a substance increases the later attractiveness of another substance. However, in people where such direct experimentation is not possible, longitudinal studies can be used to determine whether the likelihood of using one substance is related to prior use of other substances.
In mice, nicotine increased the likelihood of later use of cocaine , and the experiments led to concrete conclusions about the underlying molecular changes in the brain. The biological imprint in mice thus corresponded to the epidemiological observations that nicotine consumption in humans is linked to a later increased likelihood of cannabis and cocaine use.
In rats, cannabis increased the subsequent self-administration of nicotine in subsequent experiments. A study of the drug use of around 14,500 12th grade students showed that alcohol consumption was associated with an increased likelihood of later use of tobacco, cannabis and other illegal substances.
use
Medical use
Nicotine is used in smoking cessation therapy in the form of patches, sprays or chewing gum. The supplied nicotine reduces the withdrawal symptoms when not smoking; many of the risks posed by tobacco smoke are avoided by using pure nicotine.
A meta-analysis of 103 randomized, placebo-controlled studies found that the likelihood of relapse among smokers who quit smoking without aids is 97 percent within six months of quitting. Up until 2012, it was assumed that nicotine replacement preparations with the correct dosage and further professional guidance could increase the chances of success by three percent. A recent study from 2012 found that relapse rates among those who used nicotine replacement supplements to quit were just as high as those who quit without aids.
Nicotine chewing gums usually have a nicotine content of 2 mg for smokers with moderate tobacco consumption or 4 mg for those who are heavily dependent. In Germany they are only available in pharmacies. In Switzerland, all nicotine cessation drugs are in dispensing category D, so they are available in pharmacies and drugstores.
Regarding conjugate vaccines with nicotine to generate anti-nicotine antibodies there are similar studies. Furthermore, antagonists of the nicotinic acetylcholine receptor for weaning are being investigated.
Application in crop protection
Pure nicotine was previously used in crop protection as a pesticide against sucking or biting insects (including aphids ). The substance is well tolerated by plants and also readily biodegradable. However, due to its high toxicity, nicotine has been banned from use since the 1970s. Synthetic insecticides such as E605 were used as replacements. Other natural nicotinoids and synthetic neonicotinoids are being developed as insecticides primarily for commercial use.
Application in the e-cigarette
Nicotine is also used as an optional ingredient in the e-cigarette .
Trade names
Nicopatch (A), Nicorette (D, A, CH), Nicotinell (D, A, CH), Nicotrol (A), Nikaloz (A), Nikofrenon (D), NiQuitin (D, A)
literature
- Helmut Schievelbein (Ed.): Nicotine - Pharmacology and Toxicology of Tobacco Smoke . Thieme Verlag, Stuttgart 1968, DNB 457705825 .
Web links
Individual evidence
- ↑ a b c d e f g Entry on nicotine in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d A. C. Moffat (Ed.): Clarke's Isolation and Identification of Drugs. 2nd Edition. The Pharmaceutical Press, London 1986, ISBN 0-85369-166-5 , pp. 807-808.
- ↑ Entry on nicotine. In: Römpp Online . Georg Thieme Verlag, accessed on April 12, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-386.
- ↑ Entry on Nicotine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 54-11-5 or nicotine ), accessed on November 2, 2015.
- ↑ a b c d Entry on nicotine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Doris Schwarzmann-Schafhauser: Nicotine. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 1053.
- ↑ a b c Izuru Yamamoto, John E. Casida (Ed.): Nicotinoid insecticides and the nicotinic acetylcholine receptor . Springer, 1999.
- ↑ Entry on neonicotinoids. In: Römpp Online . Georg Thieme Verlag, accessed on September 11, 2013.
- ^ A b Dietrich Hoffmann, Ilse Hoffmann: Chemistry and Toxicology . (PDF). In: Smoking and Tobacco Control Monograph No. 9 - Cigars: Health Effects and Trends. Chapter 3, National Cancer Institute, 1998.
- ↑ H. P Rang u. a .: Rang and Dale's Pharmacology. 6th edition. Elsevier, 2007, ISBN 978-0-7020-4074-0 , p. 598.
- ^ Robert L. Metcalf: Insect Control. In: Ullmann's Encyclopedia of Industrial Chemistry . 7th edition. Wiley 2007, p. 9.
- ↑ Eckart Eich: Ornithine-Derived Alkaloids. In: Solanaceae and Convolvulaceae: Secondary Metabolites - Biosynthesis, Chemotaxonomy, Biological and Economical Significance (A Handbook). Springer Verlag, 2008, ISBN 978-3-540-74540-2 , pp. 33-188.
- ^ The Nicotine Content of Common Vegetables . In: The New England Journal of Medicine . tape 329 , August 1993, p. 437 , doi : 10.1056 / NEJM199308053290619 .
- ^ Rainer Tölle, Gerhard Buchkremer: Cigarette smoking: epidemiology, psychology, pharmacology and therapy. 2nd edition, Springer, 2013. ISBN 978-3-642-74044-2 . P. 45.
- ^ Rainer Tölle, Gerhard Buchkremer: Cigarette smoking: epidemiology, psychology, pharmacology and therapy. 2nd edition, Springer, 2013. ISBN 978-3-642-74044-2 . P. 36.
- ↑ Alfred Lichtenschopf: Standards of Tobacco Weaning: Consensus of the Austrian Society for Pneumology - Update 2010. Springer-Verlag, 2012, ISBN 978-3-7091-0979-3 , p. 55.
- ↑ On the effect of snuff , doi: 10.1007 / BF01865916 .
- ↑ Tobacco (leaf tobacco). Transportation Information Service, accessed August 13, 2017 .
- ^ A. Pinner, R. Wolffenstein: About nicotine. In: Reports of the German Chemical Society. 24, 1891, pp. 61-67, doi: 10.1002 / cber.18910240108 .
- ^ A. Pinner, R. Wolffenstein: About nicotine. In: Reports of the German Chemical Society. 24, 1891, pp. 1373-1377, doi: 10.1002 / cber.189102401242 .
- ^ A. Pinner, R. Wolffenstein: About nicotine. In: Reports of the German Chemical Society. 25, 1892, pp. 1428-1433, doi: 10.1002 / cber.189202501214 .
- ^ Paul M. Dewick: Medicinal Natural Products: A Biosynthetic Approach . 2nd edition. Wiley-Blackwell, 2001, p. 313.
- ↑ Xuewen Wang, Jeffrey L. Bennetzen: Current status and prospects for the study of Nicotiana genomics, genetics, and nicotine biosynthesis genes. In: Molecular Genetics and Genomics. 290, 2015, p. 11, doi: 10.1007 / s00438-015-0989-7 . PMID 25582664 .
- ↑ M. Iwai, T. Ogawa, H. Hattori, K. Zaitsu, A. Ishii, O. Suzuki, H. Seno: Simple and rapid assay method for simultaneous quantification of urinary nicotine and cotinine using micro-extraction by packed sorbent and gas chromatography-mass spectrometry. In: Nagoya J Med Sci. 75 (3-4), Aug 2013, pp. 255-261. PMID 24640182
- ↑ KB Scheidweiler, DM Shakleya, MA Huestis: Simultaneous quantification of nicotine, cotinine, trans-3'-hydroxycotinine, norcotinine and mecamylamine in human urine by liquid chromatography-tandem mass spectrometry. In: Clin Chim Acta. 413 (11-12), Jun 14, 2012, pp. 978-984. PMID 22394455
- ^ A. Lopes, N. Silva, MR Bronze, J. Ferreira, J. Morais: Analysis of cocaine and nicotine metabolites in wastewater by liquid chromatography-tandem mass spectrometry. Cross abuse index patterns on a major community. In: Sci Total Environ. 487, Jul 15, 2014, pp. 673-680. PMID 24200094
- ↑ N. Liachenko, A. Boulamery, N. Simon: Nicotine and metabolites determination in human plasma by ultra performance liquid chromatography-tandem mass spectrometry: a simple approach for solving contamination problem and clinical application. In: Fundam Clin Pharmacol. 29 (5), Oct 2015, pp. 499-509. PMID 26118829
- ↑ C. Müller, F. Vetter, E. Richter, F. Bracher: Determination of caffeine, myosmine, and nicotine in chocolate by headspace solid-phase microextraction coupled with gas chromatography-tandem mass spectrometry. In: J Food Sci. 79 (2), Feb 2014, pp. T251-T255. PMID 24446916
- ↑ Offermanns S .: Tabakrauch , In: Pharmakologie und Toxikologie. Springer-Lehrbuch (2016), pp. 925-929; doi: 10.1007 / 978-3-662-46689-6_72 Springer, Berlin, Heidelberg; ISBN 978-3-662-46688-9
- ↑ KDBrunnemann, D. Hoffmann: The pH of tobacco smoke , Food and Cosmetics Toxicology, Vol. 12, Issue 1, 1974, pp. 115-124, doi: 10.1016 / 0015-6264 (74) 90327-7
- ↑ Aschenbrenner, Stahl: Handbuch des Tobakhandel , 6th edition 1950, Vlg. Oldenburger Verlagshaus, p. 516 f
- ↑ J. Le Houezec: Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. In: Int J Tuberc Lung Dis . 7, 2003, pp. 811-819. PMID 12971663 .
- ↑ M. Sabha, JE Tanus-Santos, JC Toledo, M. Cittadino, JC Rocha, H. Moreno: Transdermal nicotine mimics the smoking-induced endothelial dysfunction . In: Clinical Pharmacology and Therapeutics . tape 68 , no. 2 , August 2000, p. 167-174 , doi : 10.1067 / mcp.2000.108851 , PMID 10976548 .
- ^ F. Scott Hall, Andre Der-Avakian, Thomas J. Gould, Athina Markou, Mohammed Shoaib, Jared W. Young: Negative affective states and cognitive impairments in nicotine dependence. In: Neuroscience & Biobehavioral Reviews. 2015, doi: 10.1016 / j.neubiorev.2015.06.004 . PMID 26054790 .
- ↑ Sybren F. de Kloet, Huibert D. Mansvelder, Taco J. De Vries: cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. In: Biochemical Pharmacology. 2015, doi: 10.1016 / j.bcp.2015.07.014 . PMID 26208783 .
- ↑ Otto-Michael Lesch, Henriette Walter: Alcohol and Tobacco: Medical and Sociological Aspects of Use, Abuse and Dependency. Springer-Verlag, 2009, ISBN 978-3-211-48634-4 , p. 144.
- ↑ T. Mündel, DA Jones: Effect of transdermal nicotine administration on exercise endurance in men . In: Experimental Physiology . tape 91 , no. 4 , July 2006, p. 705-713 , doi : 10.1113 / expphysiol.2006.033373 , PMID 16627574 .
- ↑ JK Cataldo, JJ Prochaska, SA Glantz: Cigarette smoking is a risk factor for Alzheimer's Disease: an analysis controlling for tobacco industry affiliation. In: Journal of Alzheimer's disease: JAD. Volume 19, Number 2, 2010, pp. 465-480, doi: 10.3233 / JAD-2010-1240 . PMID 20110594 , PMC 2906761 (free full text).
- ↑ M. Mehta, A. Adem, MS Kahlon, MN Sabbagh: The nicotinic acetylcholine receptor: smoking and Alzheimer's disease revisited. In: Frontiers in Bioscience . 2012, E4, pp. 169-180. PMID 22201862 , doi: 10.2741 / 367 .
- ↑ Maryka Quik, Xiomara A. Perez, Tanuja Bordia: Nicotine as a potential neuroprotective agent for Parkinson's disease. In: Movement Disorders. 27, 2012, p. 947, doi: 10.1002 / mds.25028 . PMID 22693036 . PMC 3685410 (free full text).
- ↑ Virender K Rehan, Jie Liu et al. a .: Perinatal nicotine exposure induces asthma in second generation offspring. In: BMC Medicine . 10, 2012, p. 129, doi: 10.1186 / 1741-7015-10-129 .
- ^ A b George E. Barreto, Alexander Iarkov, Valentina Echeverria Moran: Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease. In: Frontiers in Aging Neuroscience. 6, 2015, doi: 10.3389 / fnagi.2014.00340 . PMID 25620929 . PMC 4288130 (free full text).
- ^ YS Mineur, MR Picciotto: Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis . In: Trends Pharmacol. Sci. tape 31 , no. December 12 , 2010, p. 580-586 , doi : 10.1016 / j.tips.2010.09.004 , PMID 20965579 , PMC 2991594 (free full text).
- ^ Offermanns S. "Tobacco Smoke". In: Pharmakologie und Toxikologie , Springer-Lehrbuch (2016), pp. 925-929; doi: 10.1007 / 978-3-662-46689-6_72 Springer, Berlin, Heidelberg; ISBN 978-3-662-46688-9
- ↑ Niklaus spoon: "drug interactions with tobacco smoke" In: pharma-kritik , volume 35, PK915, online article, doi: 10.37667 / pk.2013.915
- ↑ AC Parrott: Nesbitt's Paradox resolved? Stress and arousal modulation during cigarette smoking . In: Addiction . tape 93 , no. 1 , January 1998, pp. 27-39 , doi : 10.1046 / j.1360-0443.1998.931274.x , PMID 9624709 .
- ↑ F. Pistillo, F. Clementi, M. Zoli, C. Gotti: Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: focus on nicotine effects. In: Prog Neurobiol. (2015), Volume 124, pp. 1–27. doi: 10.1016 / j.pneurobio.2014.10.002 . PMID 25447802 .
- ↑ Gaurav Jain, Edgar A. Jaimes: Nicotine signaling and progression of chronic kidney disease in smokers. In: Biochemical Pharmacology. 86, 2013, p. 1215, doi: 10.1016 / j.bcp.2013.07.014 . PMID 23892062 . PMC 3838879 (free full text).
- ^ Robert F. Smith, Craig G. McDonald, Hadley C. Bergstrom, Daniel G. Ehlinger, Jennifer M. Brielmaier: Adolescent nicotine induces persisting changes in development of neural connectivity. In: Neuroscience & Biobehavioral Reviews. 55, 2015, p. 432, doi: 10.1016 / j.neubiorev.2015.05.019 . PMID 26048001 .
- ↑ S. Zorin et al. a .: In Vitro Test of Nicotine's Permeability through Human Skin. Risk Evaluation and Safety Aspects. (PDF; 194 kB). In: Ann Occup Hyg . 43, 1999, pp. 405-413.
- ↑ B. Mayer: How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. In: Archives of Toxicology . Volume 88, Number 1, January 2014, pp. 5-7, doi: 10.1007 / s00204-013-1127-0 . PMID 24091634 . PMC 3880486 (free full text).
- ↑ D. McGee, T. Brabson, J. McCarthy, M. Picciotti: Four-year review of cigarette ingestions in children . In: Pediatric Emergency Care . tape 11 , no. 1 , 1995, p. 13-16 , doi : 10.1097 / 00006565-199502000-00004 .
- ↑ Cigarettes dangerous for small children? Swiss Toxicological Information Center.
- ↑ Frequent poisoning by nicotine. (PDF; 41 kB). Joint poison information center.
- ↑ Directive 2014/40 / EU of the European Parliament and of the Council of April 3, 2014 on the harmonization of the legal and administrative provisions of the member states on the manufacture, presentation and sale of tobacco products and related products and on the repeal of Directive 2001/37 / EG
- ↑ RAC Opinion proposing harmonized classification and labeling at EU level of Nicotine. September 10, 2015, accessed October 30, 2015.
- ↑ Regulation (EU) 2018/1480
- ^ Agents Classified by the IARC Monographs, Volumes 1-107. (PDF; 139 kB). Retrieved May 27, 2013.
- ↑ Nicotine stops chemotherapy . April 3, 2006, accessed September 8, 2019 . Wissenschaft.de.
- ↑ Piyali Dasgupta, Rebecca Kinkade, Bharat Joshi, Christina DeCook, Eric Haura, Srikumar Chellappan: Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and surviving . In: PNAS . 103, 2006, pp. 6332-6337, doi: 10.1073 / pnas.0509313103 .
- ↑ Wolfram Parzefall, Manfred Neuberger, Rolf Schulte-Hermann: The carcinogens of tobacco smoke . (PDF; 29 kB). Retrieved May 27, 2013.
- ↑ C. Schaal, SP Chellappan: Nicotine-Mediated Cell Proliferation and Tumor Progression in Smoking-Related Cancers. In: Molecular Cancer Research. 12, 2014, p. 14, doi: 10.1158 / 1541-7786.MCR-13-0541 . PMID 24398389 . PMC 3915512 (free full text).
- ↑ David J Nutt, Leslie A King, Lawrence D Phillips: Drug harms in the UK: a multicriteria decision analysis. In: The Lancet. 376, 2010, pp. 1558-1565, doi: 10.1016 / S0140-6736 (10) 61462-6 .
- ↑ Drug Toxicity. Rober Gable, accessed December 14, 2015 .
- ↑ RS Gable: Acute toxicity of drugs versus regulatory status. In: JM Fish (Ed.): Drugs and Society: US Public Policy. Rowman & Littlefield Publishers, Lanham, MD 2006, pp. 149-162.
- ^ Karl Fagerström: Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence . In: Nicotine & Tobacco Research . 14, 2012, pp. 75-78.
- ^ Anne-Sophie Villégier u. a .: Monoamine Oxidase Inhibitors Allow Locomotor and Rewarding Responses to Nicotine . In: Neuropsychopharmacology . 31, 2006, pp. 1704-1713.
- ↑ James D. Belluzzi et al. a .: Acetaldehyde Enhances Acquisition of Nicotine Self-Administration in Adolescent Rats . In: Neuropsychopharmacology. 30, 2005, pp. 705-712.
- ^ JE Rose, WA Corrigall: Nicotine self-administration in animals and humans: similarities and differences. In: Psychopharmacology . 130, 1997, pp. 28-40. PMID 9089846 .
- ↑ SCENIHR : Questions about tobacco additives: Is the development of nicotine addiction dose-dependent? (2010), accessed July 29, 2013.
-
^ L. Lewin: Fantastica. The numbing and exciting stimulants. 1924, Vlg. Georg Stilke Berlin, p. 334, footnote 1.
“There are also series of experiments in which the habituation was missed, and even after ten or hundred injections of nicotine the same phenomena repeated themselves with the same strength and with the same course. " - ↑ Surgeon General (US): How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General, Nicotine Addiction: Past and Present . 2010, accessed July 29, 2013.
- ↑ D. Nutt et al. a .: Development of a rational scale to assess the harm of drugs of potential misuse. (PDF download via Researchgate; 127 kB) 2007, accessed on July 2, 2020 .
- ↑ Harm reduction on nicotin addiction - Helping people who can't quit . ( Memento of the original from April 29, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 1.9 MB). A report by the Tobacco Advisory Group of the Royal College of Physicians, October 2007, pp. 98-99.
- ^ ER Kandel , DB Kandel : Shattuck Lecture: A molecular basis for nicotine as a gateway drug. In: The New England Journal of Medicine . Volume 371, number 10, September 2014, pp. 932-943, doi: 10.1056 / NEJMsa1405092 . PMID 25184865 , PMC 4353486 (free full text).
- ↑ KM Keyes, A. Hamilton, DB Kandel : Birth Cohorts Analysis of Adolescent Cigarette Smoking and Subsequent Marijuana and Cocaine Use. In: American Journal of Public Health . [Electronic publication before printing] April 2016, doi: 10.2105 / AJPH.2016.303128 . PMID 27077359 .
- ↑ LV Panlilio, C. Zanettini, C. Barnes, M. Solinas, SR Goldberg: Prior exposure to THC increases the addictive effects of nicotine in rats. In: Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. Volume 38, Number 7, June 2013, pp. 1198–1208, doi: 10.1038 / npp.2013.16 . PMID 23314220 , PMC 3656362 (free full text).
- ^ T. Kirby, AE Barry: Alcohol as a gateway drug: a study of US 12th graders. In: The Journal of school health. Volume 82, Number 8, August 2012, pp. 371-379, doi: 10.1111 / j.1746-1561.2012.00712.x . PMID 22712674 , PDF (accessed May 3, 2016).
- ↑ Drugs Commission of the German Medical Association: Guideline tobacco addiction from recommendations for the therapy of tobacco addiction. (PDF, 44 kB). In: Medicinal prescription in practice. Special issue. 1st edition. May 2001.
- ^ A. Molyneux: Nicotine replacement therapy. In: The BMJ . 2004, Volume 328, No. 7437, pp. 454-456. PMID 14976103 , doi: 10.1136 / bmj.328.7437.454 .
- ↑ Stead u. a .: Nicotine replacement therapy for smoking cessation. Cochrane Tobacco Addiction Group, July 16, 2008, doi: 10.1002 / 14651858.CD000146.pub3 .
- ↑ Nicotine replacement and other smoking cessation drugs . DKFZ, accessed March 6, 2013.
- ↑ HR Alpert, GN Connolly, L. Biener: A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. In: Tobacco Control . Volume 22, number 1, January 2013, pp. 32-37, doi: 10.1136 / tobaccocontrol-2011-050129 . PMID 22234781 .
- ^ EH Cerny, T. Cerny: Vaccines against nicotine. In: Hum Vaccin . 2009, Volume 5 (4), pp. 200-205. PMID 19276649 .
- ↑ PR Pentel, MG LeSage: New directions in nicotine vaccine design and use. In: Adv Pharmacol. Volume 69, 2014, pp. 553-580. doi: 10.1016 / B978-0-12-420118-7.00014-7 . PMID 24484987 ; PMC 4047682 (free full text).
- ↑ PA Crooks, MT Bardo, LP Dwoskin: Nicotinic receptor antagonists as treatments for nicotine abuse. In: Adv Pharmacol. Volume 69, 2014, pp. 513-551. doi: 10.1016 / B978-0-12-420118-7.00013-5 . PMID 24484986 ; PMC 4110698 (free full text).
- ^ Steven R. Sims: Insecticide Compositions And Process . US Patent Application 13 / 078,641.