Molecular formula
A molecular formula (also called gross formula ) is used in chemistry to indicate the type and number of atoms in a chemical compound . If the compound consists of discrete molecules , the empirical formula is a molecular formula . For a salt , a formula unit is given which is appropriate to its stoichiometric composition. A sum formula is often not the same as the ratio formula that specifies the smallest possible number ratios of the atoms of the chemical elements involved in a chemical compound.
| Structural formulas | other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
|
Electron formula |
Valence stroke formula |
Wedge formula |
Skeletal formula |
Constitutional formula |
Sum formula |
Ratio formula |
|
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
| methane |

|

|
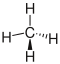
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
construction
The molecular formula of a chemical compound consists of the element symbols of the chemical elements contained and small, subscript Arabic numerals for their respective number in this compound. This number of atoms is always placed as an index to the right of the element symbols, whereby the number “1” is not written out. Instead of “H 2 O 1 ” for water , “H 2 O” is written.
If a compound consists of discrete molecules, a molecular formula indicates the composition of the molecule. The molecular formula for disulfur dichloride is S 2 Cl 2 , and for ethane C 2 H 6 . The relationship formulas are SCl and CH 3 .
There are different strategies for the order of the elements in sum formulas. In tables and databases, the Hill system is mostly preferred , especially for organic compounds . This gives first all carbon atoms, then the hydrogen atoms and sorts all other atomic symbols strictly alphabetically. This system makes it easier to find connections if you do not know the name of the connection.
For inorganic compounds, a different route is often chosen that does justice to the stoichiometric composition of the compounds: The element of higher electronegativity (usually further to the right or above in the periodic table) is usually to the right of a lower element in the empirical formula and in the name of the substance Electronegativity. For example, sodium chloride is written as “NaCl” rather than “ClNa”. For complex salts with the cation of the central atom and its is first ligand and then the anion with the central atom and its ligand specified, such as (NH 4 ) 2 SO 4 for ammonium sulfate . Inorganic acids are given analogously to the related salts. The hydrogen atoms are in front instead of the cations, as with H 3 PO 4 , the empirical formula of phosphoric acid . These formulas represent the formula units for stoichiometric calculations and not the structure of the compounds.
Examples and distinction
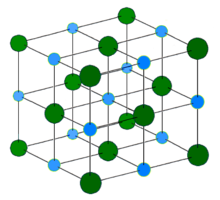
- In sodium chloride NaCl, the molar ratio of sodium to chlorine is n (Na): n (Cl) = 1: 1. Written as a size equation: n (Na) = n (Cl). The molecular formula of sodium chloride says nothing about the structure of the compound (see illustration).
- in aluminum oxide Al 2 O 3 the molar ratio n (Al): n (O) = 2: 3. As a size equation: 3 × n (Al) = 2 × n (O)
- H 2 O, a water molecule consists of two hydrogen atoms (H) and one oxygen atom (O), (atomic number ratio 2: 1);
- H 2 SO 4 , a sulfuric acid molecule, consists of two hydrogen atoms, one sulfur atom (S) and 4 oxygen atoms (atomic number ratio 2: 1: 4).
- in hexane C 6 H 14 the molar ratio n (C): n (H) = 6: 14 = 3: 7 The ratio formula of the molecule would therefore be C 3 H 7 . However, this does not represent the molecule, as it does not indicate the real atomic numbers and is therefore partly ambiguous.
In the case of simple compounds, the molecular / sum formula is often the same as the ratio formula , which specifies the smallest possible numerical ratio.
On the meaning of empirical formulas
Molecular formulas are also used in the formulation of chemical reaction equations. The starting and end products of the reaction (reactants and products) are in reaction scheme in inorganic chemistry usually given in the form of empirical formulas. These form the basis for stoichiometric calculations . In organic chemistry empirical formulas are used only rarely, since they hardly contain information that is important for the course of reactions in organic chemistry.
See also
Individual evidence
- ↑ Entry on empirical formula. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Entry on molecular formula . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03987 Version: 2.3.1.
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexikon der Chemie. Spectrum Academic Publishing House, Heidelberg, 2001.
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 433.