Valence stroke formula
The valence structural formula is a concept from chemistry in which chemical bonds are illustrated in two dimensions. Lines between the atoms represent single or multiple bonds in a molecule . It is used to provide a basic understanding of the structure of simple molecules. Molecules with an atomic bond are still represented with this type of structural formula and reaction mechanisms are illustrated with it. Sometimes the bond angles are neglected in the representation of molecules in the valence line formula, but often they are also drawn in. There are also mixed forms of both modes of representation.
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |

|

|
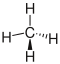
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
history
The method proposed in 1923 by Gilbert Newton Lewis in his work "Valence and the Structure of Atoms and Molecules" proved to be very successful in describing and representing the bonds between two atoms. The concept of the structural formula was not new, but Lewis first developed a systematic approach to the arrangement of atoms in molecules. While it is very often successful with diatomic molecules, especially with regard to the first two periods in the PSE , the theory originally proposed by Lewis could neither explain the geometry of the bonds nor the structure of more complicated molecules.
In spite of this, the valence line formula has been preserved in that the methods developed later were incorporated into symbolism through special conventions (e.g. mesomerism , long bond structures , multi-center bonds ). Today the valence line formula is taught in its original form in schools in order to allow an easy introduction to the more precise structure of the molecules, since many of the later developed methods can be built from the standpoint of the theory developed by Lewis.
application
Based on Bohr's atomic model or the shell model , only the outermost electrons ( outer electrons ) of the atom, i.e. the valence electrons , are taken into account when using the valence line formula . All inner electrons have no influence, just like the structure of the atomic nucleus. The use of the valence formula is essentially limited to the elements from the fourth main group (group 14 according to IUPAC nomenclature) that tend to form covalent bonds , as well as hydrogen and compounds that are built up from molecules .
The valence electrons of an atom are symbolized as points and grouped in the four cardinal directions around the element symbol of the atom. Now at least one electron of one atom is connected to one of the binding partner by a line. Now electrons that are not connected by a line count only to the atom around whose element symbol they are grouped ( free electron pair ). The electrons in the bond line are counted equally to both atoms, they are assigned to both atoms together and, so to speak, used jointly by both atoms ( binding electron pair ). Electrons of the atom are connected with those of the binding partner by a line until each of the two atoms has reached the valence electron number of the noble gases by sharing these electrons with the other atom . ( Noble gas rule ) The atom in question now has the electron configuration of the noble gas that is closest to it.
successes
The valence line formula can be used to describe most of the non-radical molecules made up of two atoms. Examples are hydrogen , nitrogen and the halogens . Molecules made up of more than two atoms can also often be noted in the valence formula. Examples are water , carbon dioxide and most organic compounds .
to fail
The magnetic behavior is often not taken into account in the valence line formula (see oxygen ). The valence bar formula reaches its limits with hypervalent molecules, i.e. with those molecules that obviously do not meet the octet rule. The sulfate ion is classically described with two double bonds and two single bonds, which would result in 12 electrons in the outer shell of the sulfur atom ( octet expansion ). In principle, the valence line formula is not suitable for predicting the spatial structure of molecules, but the spatial structure can be indicated in a similar way to the wedge line formula if it is known. The VSEPR model is often a suitable method for capturing the molecular geometry .
Alternative spellings
In addition to the valence line formula, there are other ways to represent a molecule in two dimensions:
- Electron formula , electrons are only dotted, no pairs are connected as lines
- Skeletal formula , abbreviated form in organic chemistry
- Fischer projection to draw chiral compounds like sugar
- Haworth projection , for 5- to 6-ring molecules such as sugar
- Natta projection to the stereochemistry display
- Newman projection , to show the conformation
Individual evidence
- ↑ Entry on line formula . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.L03561 Version: 2.1.5.
literature
- Gilbert Newton Lewis: Valence and the structure of atoms and molecules. 1923 , ISBN 0486615553
- Gilbert Newton Lewis: The Atom and the Molecule. J. Am. Chem. Soc. 1916 , 38, 762-785. doi : 10.1021 / ja02261a002


