hydrogen
| properties | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||
| Name , symbol , atomic number | Hydrogen, H, 1 | ||||||||||||||||||||||||
| Element category | Non-metals | ||||||||||||||||||||||||
| Group , period , block | 1 , 1 , s | ||||||||||||||||||||||||
| Appearance | colorless gas (H 2 ) | ||||||||||||||||||||||||
| CAS number | 1333-74-0 | ||||||||||||||||||||||||
| EC number | 215-605-7 | ||||||||||||||||||||||||
| ECHA InfoCard | 100.014.187 | ||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.15% | ||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||
| Atomic mass | 1.008 (1.00784-1.00811) u | ||||||||||||||||||||||||
| Atomic radius (calculated) | 25 (53) pm | ||||||||||||||||||||||||
| Covalent radius | 31 pm | ||||||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||||||
| Electron configuration | 1 s 1 | ||||||||||||||||||||||||
| 1. Ionization energy | 13.598 434 49 (8) eV ≈ 1 312.05 kJ / mol | ||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||
| Physical state | gaseous (H 2 ) | ||||||||||||||||||||||||
| density | 0.0899 kg m −3 at 273 K. | ||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −2.2 10 −9 ) | ||||||||||||||||||||||||
| Melting point | 14.01 K (−259.14 ° C) | ||||||||||||||||||||||||
| boiling point | 21.15 K (−252 ° C) | ||||||||||||||||||||||||
| Molar volume | (solid) 11.42 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||
| Heat of evaporation | 0.90 kJ / mol | ||||||||||||||||||||||||
| Heat of fusion | 0.558 kJ mol −1 | ||||||||||||||||||||||||
| Speed of sound | 1270 m s −1 at 298.15 K. | ||||||||||||||||||||||||
| Specific heat capacity | 14304 J kg −1 K −1 | ||||||||||||||||||||||||
| Thermal conductivity | 0.1805 W m −1 K −1 | ||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||
| Oxidation states | +1, 0, −1 | ||||||||||||||||||||||||
| Normal potential | 0 V | ||||||||||||||||||||||||
| Electronegativity | 2.2 ( Pauling scale ) | ||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||
Hydrogen is a chemical element with the symbol H (for Latin hydrogenium "water producer "; from ancient Greek ὕδωρ hydōr " water " and γίγνομαι gignomai "will, arise") and the atomic number 1. In the periodic table it is in the 1st period and the 1st . IUPAC group .
Hydrogen is the most common chemical element in the universe, but not in the earth's crust. It is part of water and almost all organic compounds . Thus, bound hydrogen occurs in all living organisms.
Hydrogen is the chemical element with the lowest atomic mass . Its most common isotope , also known as protium , does not contain a neutron , but consists of just one proton and one electron . Under conditions that normally prevail on earth (see also normal conditions ), this atomic hydrogen H does not occur, but the molecular hydrogen H 2 , a colorless and odorless gas . In certain chemical reactions, hydrogen appears temporarily atomically as H, referred to as nascent hydrogen . In this form it reacts particularly strongly with other compounds or elements.
history

Hydrogen was discovered by the English chemist and physicist Henry Cavendish in 1766 when he was experimenting with metals ( iron , zinc and tin ) and acids. Cavendish called the case resulting gas because of its flammability "inflammable air" ( combustible air ). He examined the gas in detail and published his findings on it that same year. However, Théodore Turquet de Mayerne (around 1620) and Robert Boyle (around 1670) produced oxyhydrogen in a similar way (action of acids on metals) as early as the 17th century .
A more detailed analysis was carried out by Antoine Laurent de Lavoisier , who called hydrogen a “water-generating substance” or “hydrogen”, giving it its current name. Cavendish had in the meantime recognized an observation by Joseph Priestley that the combustion of hydrogen produces water (published only in 1784). Lavoisier learned of Cavendish's experiments during a visit from his assistant Charles Blagden in 1783. Cavendish was a follower of the phlogiston theory and his hydrogen was a candidate for this hypothetical substance for him. Lavoisier showed in sensational experiments that it was an independent element and part of water, which at that time was often still considered to be elementary according to the old four-element theory . Lavoisier carried out his experiments quantitatively using the conservation of mass postulated by him. He conducted water vapor in a closed apparatus over red-hot iron filings and let it condense elsewhere . He found that the mass of the condensed water was slightly less than that of the original amount. For this, a gas was created whose mass, together with the increase in weight of the oxidized iron, exactly corresponded to the amount of water "lost". So his actual experiment was successful.
Lavoisier examined the resulting gas further and carried out what is now known as the oxyhydrogen test, which burned the gas. He therefore initially called it, like Cavendish, as combustible air (in French in the reverse word “air inflammable”). When he showed in further experiments that, conversely, water can be produced from the gas, he named it hydro-gène (Greek: hydro = water; genes = generating). The word therefore means: "water producer". The German name suggests the same origin of the term.
After oxygen had long been held responsible for the acidic character according to the Lavoisier school, this changed when Humphry Davy represented hydrogen chloride in 1808 and proved that it contained no oxygen. It was then recognized that instead of oxygen, hydrogen was responsible for the acidic character.
Occurrence

Hydrogen is the most common chemical element in the sun and the large gas planets Jupiter , Saturn , Uranus and Neptune , which combine over 99.99% of the mass of the solar system. Hydrogen represents 75% of the total mass or 93% of all atoms in the solar system. An even higher proportion of hydrogen is suspected in the entire universe (ignoring dark matter ).
Occurrence in the universe
Shortly after the creation of the universe, only protons and neutrons (plus electrons ) were present after the presumed destruction of the antimatter by a slight excess of matter and the condensation of a quark-gluon plasma to baryons . At the prevailing high temperatures, these combined to form light atomic nuclei, such as 2 H and 4 He. Most of the protons remained unchanged and represented the future 1 H nuclei. After about 380,000 years, the radiation density of the universe had become so low that hydrogen atoms could be formed simply by joining the nuclei with the electrons without a photon again to be torn apart.
With the continuing cooling of the universe, under the influence of gravity and based on spatial density fluctuations, clouds of hydrogen gas gradually formed, which initially clustered into large galaxies and then into protostars . Under the increasing pressure of gravity, nuclear fusion finally set in , in which hydrogen fuses to form helium. This is how the first stars and later the sun came into being.
Stars consist predominantly of hydrogen plasma . The nuclear fusion of hydrogen 1 H to helium 4 He takes place mainly via the intermediate stages deuterium 2 H and helium 3 He or via the Bethe-Weizsäcker cycle . The energy released is the energy source of the stars. The hydrogen contained in our sun makes up most of the total mass of our solar system.
The gas planets also consist largely of hydrogen. Under the extreme pressures that exist at great depths in the great gas planets Jupiter and Saturn , it can exist in metallic form . This “metallic” core is electrically conductive and presumably generates the magnetic field of the gas planets.
Outside of star systems, hydrogen also occurs in gas clouds. In the so-called HI areas , the element is molecular and non-ionized. These areas emit radiation of around 1420 MHz, the so-called 21 cm line , also known as the HI or hydrogen line , which comes from transitions in the total angular momentum. It plays an important role in astronomy and is used to locate and investigate hydrogen deposits in space.
Ionized gas clouds with atomic hydrogen, however, are called H-II areas . In these areas, stars emit large amounts of ionizing radiation. With the help of the H-II areas, conclusions can be drawn about the composition of the interstellar matter. Because of the constant ionization and recombination of the atoms, they sometimes emit visible radiation that is often so strong that you can see these gas clouds with a small telescope.
Earthly occurrences
The mass fraction is much lower on earth. In relation to the total earth mass the proportion is approx. 0.03% and in relation to the earth's crust it is approx. 2.9%. In addition, in contrast to the occurrences in space, the earthly hydrogen is predominantly bound and only rarely in pure form as an unmixed gas. The best-known connection is water. In addition to this, natural gases such as B. methane and petroleum are important hydrogen-containing compounds on earth. Hydrogen is also found in more than half of all known minerals.
Most of the hydrogen on the earth's surface occurs in the compound water. In this form it covers over two thirds of the earth's surface. The total water resources on earth amount to approximately 1.386 billion km 3 . Of this, 1.338 billion km 3 (96.5%) are salt water in the oceans . The remaining 3.5% is fresh water. Most of this is in the solid state: in the form of ice in the Arctic and Antarctic and in the permafrost soils , especially in Siberia . The small remaining portion is liquid fresh water and is mostly found in lakes and rivers, but also in underground deposits, such as groundwater.
In the earth's atmosphere , hydrogen is mainly present as gaseous water ( water vapor ). How much water vapor a unit of volume of air contains depends, in addition to the presence of water, on the air temperature. For example, air at a temperature of 30 ° C can absorb up to 4.2 percent by volume of water vapor. The relative humidity is then 100%, since the saturation vapor pressure of the water has been reached.
The abundance of molecular hydrogen in the atmosphere is only 0.55 ppm . This low proportion can be explained by the high thermal velocity of the molecules and the high proportion of oxygen in the atmosphere. At the mean temperature of the atmosphere, the H 2 particles move on average at almost 2 km / s. That is around a sixth of the escape speed on earth. Due to the Maxwell-Boltzmann distribution of the velocities of the H 2 molecules, there is still a considerable number of molecules that reach the escape velocity. However, the molecules only have an extremely short free path, so that only molecules in the upper layers of the atmosphere actually escape. More H 2 molecules follow from the layers below, and a certain proportion escapes again until ultimately only traces of the element are left in the atmosphere. In addition, the hydrogen in the lower layers of the atmosphere is burned to form water through a photo-activated reaction with oxygen. If the proportion is low, there is a balance between consumption and new production (by bacteria and photonic splitting of the water).
Extraction
Molecular hydrogen
Small amounts of hydrogen are often produced in the laboratory by the reaction of dilute acids with base metals such as e.g. B. zinc obtained. The following applies schematically:
Furthermore, molecular hydrogen can be represented by the reaction of water with alkali metals . For reasons of cost, this method is only used for demonstration tests.
The most important industrial process for the industrial production of molecular hydrogen is steam reforming . Here, hydrocarbons are reacted with water at high temperature and high pressure . This creates synthesis gas , a mixture of carbon monoxide and hydrogen. The quantitative ratio of the reaction products can then be adjusted by what is known as the water-gas shift reaction .
In the context of the power-to-gas debate, the electrolysis of water is currently gaining in importance. In water electrolysis , water is broken down into its components hydrogen and oxygen by supplying electrical energy .
- Electric current splits water into hydrogen and oxygen.
Atomic hydrogen
Atomic hydrogen can be generated by supplying the dissociation energy from the molecular element. Methodologically, this is accomplished by heating to several thousand degrees, electrical discharge at high current density and low pressure, exposure to ultraviolet light , bombardment with electrons at 10 to 20 electron volts or microwave radiation . However, atomic hydrogen (e.g. on container walls) reacts very quickly again to form molecular hydrogen. A steady state is thus established , which is usually far on the side of the molecular hydrogen.
- When energy is supplied, molecular hydrogen dissociates into its atomic form.
Wood's representation method ( Robert Williams Wood , 1898) and that of Irving Langmuir , the Langmuir torch, are particularly suitable for representing larger amounts of atomic hydrogen .
Physical Properties

Hydrogen is the element with the lowest density. Molecular hydrogen (H 2 ) is about 14.4 times less dense than air. Liquid hydrogen weighs 70.8 grams per liter. Its melting point is 14.02 K (−259 ° C), the boiling point is 21.15 K (−252 ° C). Hydrogen is poorly soluble in water and other solvents. The solubility of water is 19.4 m l / l (1.6 mg / l) at 20 ° C and normal pressure. In contrast, the solubility (more precisely the maximum volume concentration ) in metals is significantly higher.
Some thermodynamic properties (transport phenomena) are of particular importance due to the low molecular mass and the resulting high average speed of the hydrogen molecules (1770 m / s at 25 ° C) (such as the Oberth effect rocket fuel ). At room temperature, hydrogen has the highest diffusivity , the highest thermal conductivity and the highest effusion rate of all gases. Only three- or polyatomic real gases such as n -butane have a lower viscosity .
The mobility of hydrogen in a solid matrix is also very high due to the small molecular cross-section. Hydrogen diffuses through materials such as polyethylene and glowing quartz glass . A very important phenomenon is the extremely high rate of diffusion in iron, platinum and some other transition metals , as hydrogen embrittlement then occurs there. In combination with a high solubility, extremely high permeation rates occur with some materials . This results in technical uses for hydrogen enrichment, but also technical problems in the transport, storage and processing of hydrogen and hydrogen mixtures, since only hydrogen passes through these spatial limitations (see safety instructions ).
Hydrogen has a line spectrum and, depending on the temperature of the gas, a more or less pronounced continuous spectrum in the visible range. The latter is particularly pronounced in the solar spectrum. The first spectral lines in the visible range, summarized in the so-called Balmer series , are at 656 nm , 486 nm, 434 nm and 410 nm. There are also other series of spectral lines in the infrared ( Paschen series , Brackett series and Pfund Series ) and one in the ultraviolet range ( Lyman series ) of the electromagnetic spectrum . A special meaning in the radio astronomy has the 21-centimeter line (HI-line) in the hyperfine structure .
In a magnetic field , H 2 behaves very weakly diamagnetically . This means that the density of the field lines of an externally applied magnetic field decreases in the sample. The magnetic susceptibility is at standard pressure = −2.2 · 10 −9 and typically several orders of magnitude below that of diamagnetic solids.
In relation to electrical current, H 2 is an insulator. In an electric field , it has a dielectric strength of several million volts per meter.
The atomic radius of hydrogen was determined to be 37 picometers . In highly excited hydrogen atoms, see Rydberg state , as they occur under the vacuum conditions of interstellar nebulae, their electrons fly on orbits with atomic radii of up to 0.339 millimeters.
Hydrogen gas has a global warming potential of 6.
Physical states
At temperatures below 21.15 K (−252 ° C) hydrogen condenses to a clear, colorless liquid. This state is abbreviated as LH 2 (English liquid , "liquid"). Below 14.02 K (−259.2 ° C ) hydrogen forms a crystalline solid with a hexagonal close packing of spheres (hcp), there each molecule is surrounded by twelve more. At the freezing point, a sludge-like two-phase mixture, a so-called slush, forms as it cools .
In contrast to helium , when simple hydrogen ( 1 H) is liquefied, no superfluidity occurs; in principle, however, the isotope deuterium ( 2 H) can become superfluid.
The triple point of hydrogen, at which its three physical states occur simultaneously, is one of the fixed points on the international temperature scale . It is at a temperature of exactly 13.8033 K and a pressure of 7.042 kPa. The critical point is 33.18 K and 13.0 bar, the critical density is 0.03012 g / cm 3 (the lowest critical density of all elements).
Under extreme pressures such as those found in gas planets , metallic hydrogen , i.e. H. in metallic form. In doing so, it becomes electrically conductive (see conduction tape ).
Atomic and nuclear physical properties
A single hydrogen atom consists of a positively charged nucleus and a negatively charged electron , which is bound to the nucleus via the Coulomb interaction . This always consists of a single proton (main isotope 1 H) and less often, depending on the isotope, one or two additional neutrons ( 2 H or 3 H isotope ). The hydrogen atom 1 H played because of its simple construction in the development of atomic physics as a "model atom" a prominent role.
In 1913, the results of investigations into hydrogen gave rise to Bohr's atomic model , with the aid of which a comparatively simple description of many properties of the hydrogen atom is possible. One imagines that the electron revolves around the nucleus on a certain circular path. According to Bohr, the electron can also jump to other paths that are precisely defined at a distance from the nucleus, including those further out, if the necessary energy is supplied to it (e.g. through impacts in the heated gas or in the electrical gas discharge ). When jumping back from an outer to an inner path, an electromagnetic radiation or wave of a specific wavelength corresponding to the energy released is emitted. This model can be used to explain the spectral lines of the H atom that lie in visible light at wavelengths of 656 nm, 486 nm, 434 nm and 410 nm ( Balmer series ); The Lyman series is in the ultraviolet range with wavelengths of 122 nm, 103 nm, 97 nm and 95 nm. Important series in the infrared are the Paschen series (1.9 µm ; 1.3 µm; 1.1 µm and 1 µm ) and the Brackett series (4.1 µm; 2.6 µm; 2.2 µm and 1.9 µm) (only the first four lines are shown here in all series). However, Bohr's model is insufficient for the consideration of details and for other atoms to explain the observed or measured phenomena.
The quantum mechanical description, which ascribes spatially extended atomic orbitals to the electron instead of the flat Bohr orbits, is physically more correct . The hydrogen atom is the only one for which the eigenvalue problem of both the non-relativistic Schrödinger equation and the relativistic Dirac equation can be solved analytically, i.e. without the use of numerical methods . Otherwise this is only possible for the also extensively investigated hydrogen-like ions, which only have one electron left (He + , Li 2+ , etc. up to U 91+ ).
Other quantum mechanical phenomena cause further effects. The fine structure of the spectral lines comes from a. hence the orbital angular momentum and spin of the electron couple with each other. If one also takes the nuclear spin into account, one arrives at the hyperfine structure . A very small, but physically particularly interesting correction is the Lamb shift due to electromagnetic vacuum fluctuations . With all these corrections, the spectrum of hydrogen already becomes a complex phenomenon, the understanding of which requires a great deal of theoretical knowledge in quantum mechanics and quantum electrodynamics.
Nuclear spin states in the H 2 molecule
Under normal conditions, hydrogen gas H 2 is a mixture of molecules in four nuclear spin states, which differ from one another by the symmetry of their nuclear spins . They can be further differentiated into two forms of hydrogen, which are referred to as ortho and para hydrogen (o and p hydrogen for short). With o-hydrogen the nuclear spins have a symmetrical configuration, while with p-hydrogen they adopt an antisymmetrical state. o-hydrogen is the more energetic form. The two molecular states are related to one another via the following, temperature-dependent equilibrium relationship:
- The two forms can merge into one another with the absorption or release of energy.
In pure gas, equilibrium takes months at low temperatures, as the interactions between the nuclei and the shell are extremely weak. For these times, there is practically a mixture of two different gases. Despite the same chemical composition of H 2 , they even differ macroscopically due to the clearly different temperature profile of the specific heat . Apart from this, the physical properties of o- and p-hydrogen are only slightly different. For example, the melting and boiling points of the p-form are about 0.1 K below those of the o-form.
Only p-hydrogen is found at absolute zero . Since there is only one spin state for anti-parallel nuclear spins (total spin quantum number S = 0), but three states with different orientations in space for parallel nuclear spins (S = 1), one has, on the other hand, for hydrogen gas that is not too cold (about T> 200 K) im Equilibrium a ratio of para / ortho hydrogen of almost exactly 1: 3. In addition, the proportion of the o-form in the thermodynamic equilibrium cannot be increased.
In the industrial production of liquid hydrogen, the transition between o- and p-hydrogen plays an important role, because at the liquefaction temperature the equilibrium already tends strongly towards the p-form and is then quickly established at the latest in the liquid state. So that the heat that is released does not immediately allow part of the liquid obtained to evaporate again, the setting of the new equilibrium is accelerated even in the gaseous state by using catalysts .
Chemical properties
particularities
In the periodic table , hydrogen is in main group I because it has 1 valence electron . Similar to the alkali metals also found there, it has the oxidation number +1 in many compounds . However, its valence electron is on the K shell, which can only have a maximum of 2 electrons and thus the noble gas configuration is already achieved with 2 electrons and not with 8, as with the other shells.
By absorbing an electron, he can thus achieve the noble gas configuration of helium, which is only possible with very base metals. It then has the oxidation number −1 and these compounds have a halogen character ; they are called hydrides .
This position quasi "in the middle" between two noble gas configurations, in which it can accept or release the same number of electrons, is a property that is similar to the fourth main group, which explains its electronegativity , which is more like that of the also "in the middle" standing carbon than that of lithium .
Because of this "moderate" electronegativity, the bonds of hydrogen typical of main group I in the oxidation number +1 are not ionic bonds as in the case of alkali metals, but covalent molecular bonds.
In summary, the properties of hydrogen for main group I are atypical, because the fact that the K shell can only accept 2 electrons, properties of other groups are also added.
Molecular hydrogen

When ignited, hydrogen reacts violently with oxygen and chlorine , but is otherwise comparatively stable and not very reactive. At high temperatures the gas becomes reactive and forms compounds with metals and non-metals alike.
Hydrogen reacts exothermically with chlorine to form gaseous hydrogen chloride , which when dissolved in water gives hydrochloric acid . Both gases react with the same amount of substance :
- One chlorine and one hydrogen molecule each react to form two hydrogen chloride molecules
This reaction is known under the name of chlorine detonating gas reaction , which can be ignited by exposure to light. Ignition is required for the oxyhydrogen reaction (hydrogen and oxygen).
- One oxygen and two hydrogen molecules each react to form two water molecules
However, the most aggressive reaction at low temperatures is hydrogen with fluorine . If hydrogen gas is passed onto frozen fluorine at −200 ° C, the two substances react explosively with one another.
- One fluorine and one hydrogen molecule each react to form two hydrogen fluoride molecules
If the molecular hydrogen is ionized, one speaks of the dihydrogen cation . This particle occurs z. B. in low-temperature plasma discharges in hydrogen as a common ion.
- Ionization by a fast electron in the plasma
Nascent hydrogen
Hydrogen in statu nascendi , d. H. in the state of formation immediately after a hydrogen-generating reaction, exists for fractions of a second in the form of individual, very reactive H atoms. Every two of the atoms then react to form the molecule, which, however, is still in an excited state for a short time after the union . Nascent hydrogen can - in contrast to "normal" chemical behavior - cause various reactions that are not possible with molecular hydrogen.
For example, it is not possible to use hydrogen gas generated in the Kipp apparatus in an acidified, violet potassium permanganate solution (KMnO 4 ) or yellow potassium dichromate solution (K 2 Cr 2 O 7 ) to bring about the color change that indicates the reduction. This reductive color change is achieved with hydrogen in statu nascendi generated directly in these solutions by adding zinc powder .
- Nascent hydrogen can discolour purple permanganate solution under acidic conditions.
- Under acidic conditions, the yellow dichromate solution turns green due to the reducing effect of the nascent hydrogen.
Atomic hydrogen
In order to break down molecular hydrogen into atoms, energy of around 4.5 eV per molecule, or more precisely 436.22 kJ / mol (the chemist speaks of enthalpy ), must be used ; when they combine to form hydrogen molecules (H 2 ), this energy is released again:
- Two H atoms react to form one H 2 molecule and release energy in the process.
Under normal conditions, the equilibrium of this reaction is completely on the right-hand side of the equation shown, because atomic hydrogen reacts very quickly (e.g. on container walls) and strongly exothermic to molecular hydrogen (or with other reactants, if such are in the vicinity).
This reaction is used in Arcatom welding .
Molecular hydrogen is also usually present in space at low temperatures. In the vicinity of hot stars, however, molecular hydrogen is split up by their radiation, so that the atomic form predominates there. This is very reactive and quickly forms new connections, especially with other hydrogen atoms, which are, however, also split again by the radiation. See also H-II area .
Note: Hydrogen in stars is not only present in atomic form, but also as plasma : the electrons are more or less separated from the protons, depending on the temperature, due to the high temperatures that prevail there. However, the surface of the sun only has a temperature of around 6000 ° C. At this temperature most of the hydrogen is still non-ionized and even molecular, i. H. the equilibrium is far on the side of molecular hydrogen. The thermal energy at 6000 ° C is far below the energy of 4.5 eV, which is required to break the molecular bond. However, the sun is much hotter in the corona with at least one million Kelvin. Therefore, the transitions of electrons in atomic hydrogen can be seen in sunlight. Chemical compounds can hardly form at such high temperatures and disintegrate immediately.
Hydrogen bond
An important property of hydrogen is the so-called hydrogen bond , an attractive electrostatic force between two molecules. If hydrogen is bound to a strongly electronegative atom, such as fluorine or oxygen, its electron is closer to the binding partner. So there is a charge shift and the H atom now acts positively polarized. The attachment partner has a correspondingly negative effect. If two such molecules come close enough, an attractive electrical force occurs between the positive H atom of one molecule and the negative part of the respective partner. It's a hydrogen bond.
Since the hydrogen bond at only 17 kJ / mol to 167 kJ / mol is weaker than the binding force within a molecule, the molecules do not bond permanently. Rather, the hydrogen bridge only lasts for a fraction of a second because of constant movement . Then the molecules break apart to form a hydrogen bond with another molecule again. This process repeats itself over and over again.
The hydrogen bond is responsible for many properties of different compounds, such as DNA or water . In the latter case, these bonds lead to the anomalies of the water , especially the density anomaly .
Deuterium and tritium
There are three naturally occurring isotopes of hydrogen. Of all the elements, the isotopes of hydrogen differ most clearly from one another in terms of their chemical reactivity. This is due to the comparatively large difference in atomic mass ( deuterium 2 H twice, tritium 3 H three times as heavy as hydrogen 1 H).
| isotope | Surname | symbol | properties |
|---|---|---|---|
| 1 H. | Protium | H | The simplest hydrogen isotope 1 H has only one proton in the nucleus and is therefore sometimes called protium. With a relative frequency of 99.98%, it has by far the largest share of the hydrogen occurring on earth. It is not radioactive , so it is stable. |
| 2 H | deuterium | D. | The isotope 2 H has a neutron in its nucleus in addition to the proton. It is known as deuterium. For deuterium there is the D as a separate element symbol . It is used e.g. B. as a component of solvents for the 1 H-NMR spectroscopy, since it does not provide a disturbing secondary signal. It makes up 0.015% of all hydrogen atoms. Deuterium is also stable. |
| 3 H | Tritium | T | Tritium is the third naturally occurring isotope of hydrogen. However, it only makes up a negligible proportion of the total naturally occurring hydrogen. Tritium has two neutrons and is marked with 3 H or T. Tritium is radioactive and breaks down into 3 He through beta decay (β - ) with a half-life of 12.32 years . Tritium is continuously formed as a cosmogenic radionuclide by nuclear reactions in the upper atmosphere . With a balance of natural production and decay, according to the source, there is an inventory of 3.5 kg on earth. Tritium can be detected in surface water and in living things. |
| 4 H, 5 H, 6 H, 7 H | Recent evidence. All isotopes have very short lifetimes (<10 −21 s). |
Hydrogen-like isotopes
By including muons , negatively charged unstable elementary particles with about 10% of the mass of a proton, exotic short-lived structures can be created that behave chemically like a hydrogen atom. Since muons rarely occur naturally and their lifespan is only 2 µs, such hydrogen isotopes are artificially produced in particle accelerators .
The muonium consists of an electron and a positively charged antimuon , which takes on the role of the proton (i.e. the atomic nucleus). Because of its atomic number of 1 e, muonium is chemically hydrogen. Because of the low atomic mass of 0.1 u (1/10 of H), isotope effects are particularly pronounced in chemical reactions, so that theories for reaction mechanisms can be checked well.
An exotic hydrogen with a mass of 4.1 u is created when one of the electrons in a 4 He atom is replaced by a muon . Due to its much higher mass compared to the electron, the muon is located close to the He nucleus and shields one of the two elementary charges of the nucleus. Together, the He nucleus and muon effectively form a nucleus with a mass of 4.1 u and a charge of 1 e, so it is chemically hydrogen.
use
Every year, more than 600 billion cubic meters of hydrogen (around 30 million t) are extracted for countless applications in industry and technology. Important areas of application are:
- Energy carrier : When welding, as rocket fuel . Its use as fuel for jet engines , in hydrogen combustion engines or via fuel cells is expected to be able to replace the use of petroleum products in the foreseeable future (see hydrogen drive), because the combustion primarily produces water, but no soot and no carbon dioxide . However, unlike petroleum, hydrogen is not a primary energy .
- Carbohydrate : Through various chemical reactions, coal is convertedinto liquid hydrocarbons with H 2 . In this way, petrol , diesel and heating oil can be produced artificially.
At the moment, both of the aforementioned processes are not yet of any economic importance due to their higher costs. But that could change drastically once the earth's oil supplies run out.
- Reducing agent : H 2 can react with metal oxides and thereby deprive them of oxygen. Water and the reduced metal are created. The process is used in the smelting of metallic ores , in particular to extract metals as pure as possible.
- Ammonia production : The Haber-Bosch process is used toproduce ammoniafrom nitrogen and hydrogen and use it to produce important fertilizers and explosives.
- Fat hardening : hardened fats are obtained from vegetable oil by means of hydrogenation . The double bonds in unsaturated fatty acid residues of the glycerides are saturated with hydrogen. The resulting fats have a higher melting point, which makes the product solid. This is the way to make margarine . This can also result in harmful trans fats as a by-product.
- Food additive : Hydrogen is approved as E 949 and is used as a propellant, packing gas, etc. used.
- Coolant : Due to its high heat capacity , (gaseous) hydrogen is used in power plants and the turbo generators used there as a coolant. In particular, H 2 is used where liquid cooling can be problematic. The heat capacity comes into play where the gas cannot circulate or can only circulate slowly. Because the thermal conductivity is also high, flowing H 2 is also used to transport thermal energy into large reservoirs (e.g. rivers). In these applications, hydrogen protects the systems from overheating and increases efficiency. The advantage here is that hydrogen, due to its low density, which is included in the Reynolds number, flows in a laminar manner up to higher speeds with little resistance than other gases.
- Cryogenic : Due to its high heat capacity and low boiling point, liquid hydrogen is suitable as a cryogen, i.e. as a coolant for extremely low temperatures. Even larger amounts of heat can be absorbed well by liquid hydrogen before a noticeable increase in its temperature occurs. In this way, the low temperature is maintained even with external fluctuations.
- Carrying gas :Hydrogen was first usedin balloons and airships . Because of thehighflammability of H 2 -air mixtures, however, this repeatedly led to accidents. The biggest catastrophe in this context is probably the "Dixmude" disaster in1923, the most famous was certainly the "Hindenburg catastrophe" in 1937. Hydrogen as the lifting gas has now been replaced by helium and only fulfills this purpose in very special applications.
The two natural isotopes have special areas of application.
Deuterium is used (in the form of heavy water ) in heavy water reactors as a moderator , i. H. to slow down the fast neutrons produced during nuclear fission to thermal speed.
Deuterated solvents are used in nuclear magnetic resonance spectroscopy because deuterium has a nuclear spin of one and is not visible in the NMR spectrum of the normal hydrogen isotope.
In chemistry and biology, deuterium compounds help in the investigation of reaction processes and metabolic pathways ( isotope marking ), since compounds with deuterium usually behave almost identically in chemical and biochemical terms as the corresponding compounds with hydrogen. The reactions are not disturbed by the labeling, but the whereabouts of the deuterium can still be determined in the end products.
Furthermore, the considerable difference in mass between hydrogen and deuterium ensures a clear isotope effect in the mass-dependent properties. So that has heavy water a measurably higher boiling point than water.
The radioactive isotope tritium is produced in nuclear reactors in industrially usable quantities. In addition to deuterium, it is a starting material for nuclear fusion to form helium. In civilian use, it is used as a radioactive marker in biology and medicine. In this way, for example, tumor cells can be detected. In physics it is, on the one hand, a subject of research; on the other hand, heavy nuclei are investigated with highly accelerated tritium nuclei or artificial isotopes are produced.
With the help of the tritium method , water samples can be dated very precisely. With a half-life of around twelve years, it is particularly suitable for measuring relatively short periods of time (up to a few hundred years). Among other things, the age of a wine can be determined in this way.
It is used as a long-lasting, reliable energy source for luminous colors (mixed with a fluorescent dye ), especially in military applications, but also in wristwatches. The isotope is also used for military purposes in the hydrogen bomb and certain versions of nuclear weapons , the effect of which is based on fission.
Hydrogen as an energy store
Hydrogen is considered an energy carrier of the future.
Production of hydrogen
(→ See also main article: Hydrogen production )
As an energy carrier , hydrogen - like electrical energy - is not a primary energy , but has to be produced from primary energy like electricity .
Hydrogen as an energy carrier does not cause carbon dioxide if it is obtained with renewable energies such as wind energy or solar energy . Also biohydrogen does not cause carbon dioxide in the net balance. Currently (2019), however, hydrogen is produced almost exclusively from fossil primary energy, mainly through natural gas reforming .
The production by water electrolysis with excess renewable electricity, often favored under the catchphrase " power-to-gas ", is relatively inefficient and economically not competitive compared to reforming natural gas because the electricity surplus is sufficiently cheap , with efficiencies of barely over 60% can actually only be used for a few hours a year and, with such low utilization, the necessary system technology can only be financed with high subsidies in research and pilot systems . That can only change if a future electricity supply that has been converted to a predominantly regenerative power supply yields significantly more surpluses that cannot be used otherwise, or if natural gas as a raw material becomes more expensive than regenerative power generation or is subject to a correspondingly high CO 2 emission .
(→ See also section: Technologies for hydrogen production)
Storage of hydrogen
(→ See also main article: Hydrogen storage )
Hydrogen contains more energy per mass than any other fuel: 141.8 MJ / kg ≈ 39.39 kWh / kg calorific value corresponds to that of 4.4 liters or 3.3 kg petrol. Conversely, the energy content per volume is relatively low and even in the liquid state reaches only 10 MJ / L ≈ 2.79 kWh / L, which is only 31% of the energy per liter of gasoline. Hydrogen therefore requires large and heavy tanks.
The technical problems associated with storing hydrogen arise primarily from its high vapor pressure, its low boiling point and its high tendency to diffuse. Processes such as pressurized and liquid hydrogen storage and storage in metal hydrides are in commercial use. In addition, there are other processes that are still in the development stage or in basic research. (→ See also section: Hydrogen storage technologies)
The following storage methods are used:
- Storage as cryogenic liquid hydrogen in vacuum-insulated containers (14.12 L / kg at 20 K ≈ −253 ° C); highest possible storage density, liquefaction energy-intensive. Some gas is constantly escaping.
- Storage of gaseous hydrogen in high-pressure containers (55 L / kg at 200 bar to 25 L / kg at 700 bar, 15 ° C); no cooling or thermal insulation necessary, diffusion losses can occur.
- Storage of hydrogen at lower pressure, bound in metal hydrides , carbon nanotubes or liquid organic hydrogen carriers (LOHC); higher security, handling simplified. A 200 kg tank can only store around 2 kg of hydrogen (corresponds to approx. 9 liters of gasoline). The hydrogen has to be partially released from the bond by supplying heat in order to be able to use the full capacity.
High-pressure tanks made of carbon fiber reinforced plastic for up to 800 bar, for example, hold 125 liters of hydrogen at 700 bar nominal pressure and only 5 kg of hydrogen (equivalent to 22 liters of gasoline) and weigh around 125 kg.
Fire load & risk of explosion
Pure hydrogen in the tank is not explosive without oxygen. In the event of overpressure due to overheating, hydrogen is vented in a metered manner via safety valves on the tank and evaporates quickly. An ignition source can ignite escaping hydrogen, which then flares up rapidly without causing an explosion. The high volatility of hydrogen makes an explosion in the open air very unlikely. If larger quantities escape in closed rooms (e.g. in the event of a fire in a garage), however, the formation of an explosive air mixture from 4 volume percent is conceivable. With a low storage capacity of a maximum of 5 kg of hydrogen with a calorific value of approx. 600 MJoules , which is typical for FCHV cars , the fire load is always lower than the calorific value of 20 liters of petrol, which is also much more dangerous if it is splashed by an impact or spread over a large area spreads across the ground instead of immediately escaping upwards like hydrogen gas.
Energy densities in comparison
| fuel | Calorific value / mass | density | Calorific value / vol | calorific value |
|---|---|---|---|---|
| hydrogen | 39.39 kWh / kg = 141.8 MJ / kg | 0.090 kg / m³ | 3.54 kWh / m³ = 12.7 MJ / m³ | ≈ 85% ≙ 3 kWh / m³ = 11 MJ / m³ |
| Methane CH 4 | 13.9 kWh / kg = 50 MJ / kg | 0.72 kg / m³ | 10 kWh / m³ = 36 MJ / m³ | ≈ 90% ≙ 9 kWh / m³ = 32 MJ / m³ |
| Natural gas "H" | 13.9 kWh / kg = 50 MJ / kg | 0.80 kg / m³ | 11.1 kWh / m³ = 40 MJ / m³ | ≈ 90% ≙ 10 kWh / m³ = 36 MJ / m³ |
| diesel | 12.5 kWh / kg = 45 MJ / kg | 0.83 kg / L | 10.5 kWh / L = 37.8 MJ / L | ≈ 94% ≙ 9.8 kWh / L = 35 MJ / L |
| petrol | 12.0 kWh / kg = 43 MJ / kg | 0.75 kg / L | 9.0 kWh / L = 32.4 MJ / L | ≈ 94% ≙ 8.5 kWh / L = 31 MJ / L |
In terms of volume:
- Hydrogen (liquid): 2360 kWh / m³
- Petrol: 8760 kWh / m³
- Natural gas (20 MPa): 2580 kWh / m³
- Hydrogen gas (20 MPa): 530 kWh / m³
- Hydrogen gas (normal pressure): 3 kWh / m³
Nuclear fusion
Soon after the beginnings of nuclear physics in the first quarter of the 20th century, the attention of physicists was drawn to energy generation. In addition to nuclear fission, the way of a fusion of the nuclei, the nuclear fusion, was explored. The first reactions found are the proton-proton reactions , in which hydrogen nuclei fuse directly to form helium. That could largely explain the energy production in light stars like our sun. Between 1937 and 1939, Hans Bethe and Carl Friedrich von Weizsäcker developed a theory of nuclear fusion in very heavy stars, the Bethe-Weizsäcker cycle named after them . Hydrogen plays the predominant role in energy generation. However, it is not fused directly to form helium, but rather fuses in various reactions with carbon, nitrogen and oxygen. At the end of the cycle, helium is produced; the other elements act as catalysts.
During the Cold War, the great powers expanded their nuclear arsenals. The US was the first to take the step towards fusion weapons: based on the atom bomb , which derives its energy from nuclear fission , American researchers under Edward Teller constructed the hydrogen bomb . In it, nuclear fusion releases a multiple of the energy of a uranium bomb. In 1952, the United States tested the first hydrogen bomb on a small Pacific island. Fuel was not hydrogen, but the isotope deuterium . The main nuclear reactions that took place in the bomb were:
The resulting tritium and helium -3 can react further:
In total, three deuterons create a helium nucleus, a neutron and a proton.
Since deuterium, like hydrogen, is difficult to store, most fusion weapons now use lithium deuteride (LiD) as a fuel. The neutrons produced in the primary reaction of deuterium produce tritium from the lithium:
- The neutron bombardment of lithium produces helium and the fusion fuel tritium.
The reaction with lithium-6 also releases energy, while the reaction with lithium-7 consumes energy, but generates a neutron again that is available for further tritium production.
Physicists are also researching the peaceful use of nuclear fusion for energy generation. Attempts to control the reaction in a plasma are the most advanced . The very high temperatures required for this are difficult to achieve. The first corresponding test facilities were set up from around 1970. Among the leading plants today (2016) are, for example, JET and ITER (under construction) in Europe, a German tokamak reactor in Garching and the Wendelstein 7-X stellarator at the Max Planck Institute for Plasma Physics (IPP) in Greifswald.
If the experiments are successful, the knowledge gained will be used to build a demonstration power plant ( DEMO ). The current planning is based on the commissioning of DEMO around 2040 and possible commercial use from around 2050. Unlike hydrogen bombs, such commercial reactors will probably only be able to use the deuterium-tritium reaction to generate energy. You are therefore absolutely dependent on lithium for breeding the actual fuel tritium. While deuterium is available in almost any quantity in the world's oceans, the known lithium reserves are limited.
Nuclear fusion in the sun and stars
With hydrogen burning the nuclear fusion of hydrogen into is helium inside stars (z. B. a Nova , on the surface of a white dwarf ), respectively. This reaction is the main source of energy in normal stars for most of their life cycle. Despite its historical name, it has nothing to do with chemical combustion.
The process of nuclear fusion can take place in two ways in hydrogen burning, in which four protons, the atomic nuclei of hydrogen, are converted into a helium nucleus 4 He in different ways:
- the relatively direct proton-proton reaction
- the Bethe-Weizsäcker cycle ( CNO cycle ) using heavy elements (carbon, nitrogen, oxygen )
For the exact calculation of the released energy, it must be taken into account that in the partial reaction of the proton-proton reaction and also the Bethe-Weizsäcker cycle, two positrons are released which, when annihilating with an electron, release 1.022 MeV corresponding to the masses of the electron and positron . To the mass difference of the four protons and the helium nucleus, two times the electron mass must therefore be added. This mass difference is identical to the difference between four times the atomic mass of protium, hydrogen consisting of protons and electrons and the atomic mass of 4 He. These atomic masses are approximately, but not exactly, identical to the atomic masses of hydrogen and helium, as there are different isotopes of these elements. Furthermore, a small part of the energy leaves the sun in the form of neutrinos .
In total, about 0.73% of the mass is converted into energy during hydrogen burning, which is known as a mass defect . The energy generated from the mass difference results from the Einstein relationship E = mc ². It results from the nuclear binding energy of the nucleons , the core building blocks.
The fusion of hydrogen to helium is most productive; the next stage of stellar fusion reactions, the burning of helium , only releases about a tenth of this energy for each carbon nucleus produced.
Biological importance
In the form of a wide variety of compounds, hydrogen is essential for all known living things. First and foremost, water should be mentioned, which serves as a medium for all cellular processes and for all material transport. Together with carbon, oxygen, nitrogen (and, more rarely, other elements), it is part of those molecules from organic chemistry without which any known form of life is simply impossible.
Hydrogen also plays an active role in the organism, for example with some coenzymes such as B. Nicotinamide adenine dinucleotide (NAD / NADH), which serve as reduction equivalents (or "proton transporters") in the body and participate in redox reactions . In the mitochondria , the power stations of the cell, the transfer of hydrogen cations (protons) between different molecules of the so-called respiratory chain serves to provide a proton gradient through chemiosmotic membrane potential to generate high-energy compounds such as adenosine triphosphate (ATP). During photosynthesis in plants and bacteria, the hydrogen from the water is needed to convert the fixed carbon dioxide into carbohydrates .
In relation to the mass, hydrogen is the third most important element in the human body : For a person with a body weight of 70 kg, around 7 kg (= 10% by weight) are due to the hydrogen contained. Only carbon (approx. 20% by weight) and oxygen (approx. 63% by weight) make up an even larger proportion by weight. In relation to the number of atoms, the very light hydrogen is by far the most common atom in the body of every living being. (The 7 kg in humans correspond to 3.5 · 10 3 mol of hydrogen with 2 · 6 · 10 23 atoms each , that is around 4.2 · 10 27 hydrogen atoms).
Medical importance
In biological systems, molecular hydrogen reacts with reactive oxygen species and thus acts as an antioxidant . In animal experiments, the enrichment of drinking water with molecular hydrogen after kidney transplantation leads to better survival of the transplant, to a reduced incidence of chronic damage to the transplant, to a reduction in the concentration of reactive oxygen species and to an inhibition of signaling pathways that increase inflammatory activity ( pro-inflammatory pathways).
Significance in competitive sport
Due to its effect as an antioxidant , hydrogen has a performance-enhancing effect during anaerobic stress. It can be used in low doses during training over a longer period of time, as well as in high doses immediately before or during the competition, e.g. B. in half-time breaks. It can be added to beverages for immediate use as well as in gaseous form similar to oxygen z. B. be inhaled through a mask. Only intravenous administration is prohibited by anti- doping regulations .
safety instructions
Hydrogen is extremely flammable. It burns with pure oxygen or air as well as with other gaseous oxidizing agents such as chlorine or fluorine with a hot flame. Since the flame is barely visible, you can get into it unintentionally. Mixtures with chlorine or fluorine are already flammable by ultraviolet radiation (see chlorine detonating gas ). In addition to the labeling required by GHS (see info box), H 2 pressurized gas cylinders according to DIN EN 1089-3 must have a red cylinder shoulder and a red cylinder body .
Hydrogen is non-toxic and does not harm the environment. Therefore, no MAK value is specified. Respiratory protection or skin protection are not required. Only when high concentrations are inhaled can movement disorders , unconsciousness and asphyxiation occur due to the lack of oxygen from around 30% by volume .
Mixtures of air and 4 to 76 % by volume of hydrogen are flammable. From a concentration of 18% in air, the mixture is explosive ( oxyhydrogen ). The ignition temperature in air is 560 ° C. When handling the hydrogen, keep it away from sources of ignition, including electrostatic discharge. The containers should be stored away from oxidizing gases (oxygen, chlorine) and other fire-promoting substances.
Due to its small atomic size, hydrogen can diffuse through many solids, i.e. gas can slowly escape through unsuitable materials (e.g. plastics). The materials and thicknesses used for gas tanks and lines take this into account so that there are no greater risks than e.g. B. with gasoline. Hydrogen vehicles with pressure tanks can easily be parked in multi-storey car parks and underground garages. There is no legal provision that restricts this ( see : hydrogen storage ).
proof
Molecular hydrogen can be detected by the oxyhydrogen sample. In this detection reaction, a small amount of a gas, for example captured during a reaction, is ignited in a test tube . If a dull bang, whistling or barking can be heard afterwards, the detection is positive (i.e. there was hydrogen in the test tube). The bang is caused by the reaction of hydrogen gas with the oxygen in the air:
- (exothermic reaction)
- Hydrogen reacts with oxygen to form water
With the same reaction, hydrogen burns with a slightly bluish flame if it is ignited at the point of exit (whistling gas).
The oxyhydrogen sample is the "classic" method of detection and is particularly popular in school experiments.
links
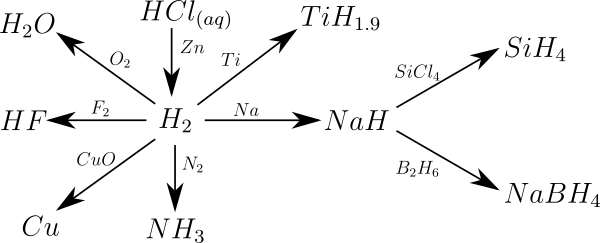
Hydrogen forms compounds with most of the chemical elements with the general empirical formula EH n ( n = 1, 2, 3, 4). A few of these hydrogen elements are only known in the form of so-called adducts , such as L m · EH n (L stands for a ligand ). The following figure provides an overview of important basic reactions of hydrogen. Precise reaction conditions and stoichiometry are not taken into account here.
Hydrogen can carry both positive and negative charges in compounds . This depends on whether the binding partner has a higher or lower electronegativity than hydrogen (2.2). No sharp boundary can be drawn in the periodic table between the two types of compounds, as, for example, the acid-base behavior must be taken into account. A more or less arbitrary consideration says that in the hydrogen compounds of the elements boron , silicon , germanium , tin and lead as well as all the hydrogen is negatively polarized to the left of it, in compounds with carbon , phosphorus , arsenic , antimony , bismuth and all elements to the right of it positive. Correspondingly, in monosilane (SiH 4 ) the oxidation number for silicon can be set to +4 (hydrogen correspondingly −1), in methane (CH 4 ) for carbon to −4 (hydrogen +1).
To represent hydrogen compounds EH n , three different methods are mainly used:
- The implementation of the corresponding element E with hydrogen (H 2 ; hydrogenolysis )
- An element reacts with hydrogen when energy is supplied to the corresponding element hydrogen.
- The reaction of metal compounds of the type M n E with hydrogen acids (H + ; protolysis )
- A metal compound of the element E reacts with an acid HA to form element hydrogen and a metal salt.
- The reaction of halogen compounds (EHal n ) with hydrides (H - ; hydridolysis )
- Hydride ions release the corresponding element hydrogen from a halogen compound of element E.
Salt-like compounds
In connection with metals, hydrogen can absorb one electron at a time, so that negatively charged hydrogen ions (hydride ions, H - ) are formed, which form salts with metal cations. These compounds are called hydrides . Salt-like hydrogen elements are known from the alkali metals and, with the exception of beryllium , the alkaline earth metals . The dihydrides of europium and ytterbium (EuH 2 and YbH 2 ) are also included.
Metal hydrides react very violently with water, releasing molecular hydrogen (H 2 ) and can self-ignite in air, with the formation of water and the metal oxide. The majority, however, are not explosive. Minerals that contain hydrogen (bound to oxygen) are hydrates or hydroxides .
Metal-like compounds
In metal-like hydrogen compounds - with a few exceptions these are the transition metal hydrides - atomic hydrogen is embedded in the corresponding metal structure. In this case one also speaks of hydrogen intercalation compounds, although the structure of the metal changes when the hydrogen is absorbed (which actually does not correspond to the definition for intercalation compounds). The element occupies the octahedral and tetrahedral gaps in the cubic and hexagonal densest metal atom packings .
The solubility of hydrogen increases with increasing temperature. However, even at temperatures above 500 ° C., more than 10 atomic percent hydrogen is rarely found in the metal in question. The elements vanadium , niobium and tantalum can absorb most of the hydrogen . The following stoichiometries can be observed at room temperature : VH 0.05 , NbH 0.11 and TaH 0.22 . A 1: 1 stoichiometry (MH) is found for these metals from 200 ° C. The body-centered cubic crystal lattice remains untouched.
Covalent bonds
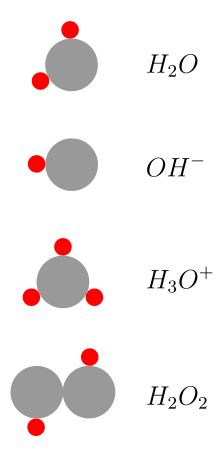
Compounds in which hydrogen is the more electropositive partner have a high covalent content. Examples are hydrogen fluoride (HF) or hydrogen chloride (HCl). In water these substances react as acids , since the hydrogen can be split off immediately as a proton (H + ion) from the surrounding water molecules. Isolated H + ions combine immediately with water molecules in aqueous solution to form H 3 O + ions ; this ion is responsible for the acidic properties of aqueous hydrogen chloride solutions.
Acid-base behavior
The covalent hydrogen compounds of the elements of the IV. To VII. Main group of the periodic table as well as boron hydrogen are acids according to the definition of Johannes Nicolaus Brønsted , i.e. they give off protons to other compounds.
The acidity of the compounds increases in the main groups from top to bottom and in the periods from left to right. It also increases with the number of element-element bonds in hydrogen bonds of a certain element. For example, water (H 2 O) is a weaker acid than hydrogen peroxide (H 2 O 2 ), ethane (C 2 H 6 ) is weaker in acid strength than ethene (C 2 H 4 ) and ethyne (C 2 H 2 ).
Conversely, covalent hydrogen elements can act as bases . Hydrogen compounds of the elements from main groups V to VII can take up protons because they have free electron pairs .
The cause of the acidity or basicity of an aqueous solution is the molar concentration of protons (H + ions). The negative decadic logarithm of this concentration is called the pH value . For example, a concentration of 0.001 mol H + ions per liter of water means “pH 3.0”. This example applies to an acid. Water without any additives has pH 7 under normal conditions , bases have pH values up to 14.
Oxides
Hydrogen oxides (also hydrogenium oxides) are compounds that consist only of hydrogen and oxygen, of the greatest importance is water (hydrogen oxide); Hydrogen peroxide , formerly known as hydrogen peroxide , is also of technical importance . Another, but rarer, oxide is dihydrogen trioxide .
Alcohols and saccharides as well as carboxylic acids, which contain (only) hydrogen, oxygen and carbon, are of extraordinary importance for all life on earth.
Hydrocarbons
Together with carbon, hydrogen forms the covalent hydrocarbons that hydrocarbon chemistry is dedicated to studying.
literature
chemistry
- Erwin Riedel : Inorganic Chemistry . de Gruyter, Berlin 2002, ISBN 3-11-017439-1 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 259-296.
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
technology
- Peter Kurzweil: Fuel cell technology. 1st edition. Vieweg Verlag, Wiesbaden 2003, ISBN 3-528-03965-5 .
- Udo Schelling: fuel cells. In: Richard Zahoransky (Ed.): Energy technology. 5th, revised. u. exp. Edition. Vieweg + Teubner Verlag, Wiesbaden 2010, ISBN 978-3-8348-1207-0 , pp. 203ff.
- Helmut Eichlseder, Manfred Klell: Hydrogen in vehicle technology. 1st edition. Vieweg + Teubner Verlag, Wiesbaden 2008, ISBN 978-3-8348-0478-5 .
- Sven Geitmann: Hydrogen & Fuel Cells - The Technology of Tomorrow. 2nd Edition. Hydrogeit Verlag, Kremmen 2004, ISBN 3-937863-04-4 .
- Rex A. Ewing: Hydrogen - A Journey Into a World of Hydrogen Energy and Fuel Cells. Pixyjack Press, Masonville CO 2004, ISBN 0-9658098-6-2 .
meaning
- Hoimar von Ditfurth : In the beginning there was hydrogen. dtv, Munich 2002, ISBN 3-423-33015-5 .
Web links
- Link catalog on hydrogen at curlie.org (formerly DMOZ )
- A hydrogen and deuterium spectral tube operating at 1.8 kV, 18 mA and a frequency of 35 kHz.
Individual evidence
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry. 8th edition. Verlag de Gruyter, 2011, ISBN 978-3-11-022566-2 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (hydrogen) unless otherwise stated .
- ↑ The standard value recommended by IUPAC is given, since the isotopic composition of this element can vary locally, the mass range given in brackets results for the mean atomic weight. See: Michael E. Wieser, Tyler B. Coplen: Atomic weights of the elements 2009 (IUPAC Technical Report). In: Pure and Applied Chemistry. 2010, p. 1, doi: 10.1351 / PAC-REP-10-09-14 .
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ Entry on hydrogen in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ Entry on hydrogen at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ a b c Entry on hydrogen in the GESTIS substance database of the IFA , accessed on May 3, 2017(JavaScript required) .
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Entry on Hydrogen in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Ernst F. Schwenk: Great moments in early chemistry. Verlag CH Beck, 1998, ISBN 3-406-45601-4 .
- ↑ Martin Carrier, Cavendishs Version der Phlogistonchemie or: On the empirical success of inaccurate theoretical approaches, in: J. Mittelstraß, Chemie und Geisteswissenschaften, Berlin, Akademie Verlag 1992, pp. 35–52, (online)
- ^ The composition of the Earth. (PDF) Retrieved December 16, 2019 .
- ↑ Webmineral - Mineral Species sorted by the element H (Hydrogen) (English).
- ↑ a b Entry on hydrogen. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.
- ↑ Entry on hydrogen in the GESTIS substance database of the IFA , accessed on December 16, 2019(JavaScript required) .
- ↑ GIT laboratory journal. Issue 9/2013, p. 596, according to Jürgen Quadbeck-Seeger (Ed.): Chemical records . Wiley-VCH.
- ↑ Global warming potential (GWP) of selected compounds and their mixtures according to the fourth assessment report of the IPCC based on a period of 100 years. (PDF) In: Umweltbundesamt.de. Retrieved September 18, 2018 .
- ↑ a b c d Entry on hydrogen . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD .
- ↑ George A. Jeffrey: An Introduction to Hydrogen Bonding . Oxford University Press, 1997, ISBN 978-0-19-509549-4 .
- ^ D. Lal, B. Peters: Cosmic ray produced radioactivity on the earth. In: Handbook of Physics. Volume 46/2, Springer, Berlin 1967, pp. 551-612.
- ↑ Belle Dumé: Hydrogen-7 makes its debut. March 7, 2003, accessed December 1, 2013 .
- ↑ a b c chemistry with unusual elementary particles , spectrum direct January 28, 2011.
- ↑ Additive Admissions Ordinance : Annex 3 (to Section 5, Paragraph 1 and Section 7) Generally permitted additives .
- ^ VDE: Hydrogen as the energy carrier of the future ( memento of October 26, 2012 in the Internet Archive ), accessed on April 11, 2012.
- ↑ https://www.energieagentur.nrw/brennstoffzelle/brennstoffzelle-wasserstoff-elektromobilitaet/produktion Information from the Energy Agency North Rhine-Westphalia on fuel cells and hydrogen: alkaline electrolysis / Lurgi process, accessed on August 11, 2020
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ^ German Hydrogen and Fuel Cell Association: DWV Hydrogen Safety Compendium ( Memento from March 17, 2014 in the Internet Archive ) (PDF; 2.1 MB), November 2011.
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ↑ energie-lexikon.info
- ↑ Jon S Cardinal, Jianghua Zhan, Yinna Wang, Ryujiro Sugimoto, Allan Tsung, Kenneth R McCurry, Timothy R Billiar, Atsunori Nakao: Oral hydrogen water prevents chronic allograft nephropathy in rats . In: Kidney International . tape 77 , no. 2 , January 2010, p. 101-109 , doi : 10.1038 / ki.2009.421 , PMID 19907413 .
- ↑ . Botek, M., Krejčí, J., McKune, AJ et al. (2019), Hydrogen Rich Water Improved Ventilatory, Perceptual and Lactate Responses to Exercise. Int J Sports Med 40 (14), 879-885.
- ↑ Aoki, K., Nakao, A., Adachi, Tusdm et al. (2012), Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2 (12). doi: 10.1186 / 2045-9912-2-12
- ^ Arnd Krüger : Hydrogen. Competitive sport . 50, (2020), 2, pp. 29-32
- ↑ a b Media Forum German Hydrogen Day, Axel Stepken: Hydrogen - As safe as gasoline. (PDF; 704 kB).
- ↑ Helmut Eichlseder, Manfred Klell: hydrogen in the vehicle art. 2010, ISBN 978-3-8348-0478-5 .
- ↑ Spectacular test shows: Hydrogen in the car doesn't have to be more dangerous than gasoline. In: Wissenschaft.de. February 3, 2003, accessed September 8, 2019 .
- ↑ Safety aspects when using hydrogen ( Memento from March 6, 2012 in the Internet Archive ) Source: Hycar.
- ↑ University of Miami crash test on YouTube