Fermium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Fermium, Fm, 100 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Actinoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | Ac , 7 , f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-72-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 257,0951 u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | - (equivalent: 198) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Rn ] 5 f 12 7 s 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.50 (7) eV ≈ 627 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 12.4 (4) eV ≈ 1 200kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 23.2 (4) eV ≈ 2 240 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 39.3 (4) eV ≈ 3 790 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 55.0 (1.9) eV ≈ 5 310 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | (calculated) 1125 K (approx. 852 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +2, +3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −1.96 V (Fm 3+ + 3 e - → Fm) −2.37 V (Fm 2+ + 2 e - → Fm) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fermium is an exclusively artificially produced chemical element with the element symbol Fm and the atomic number 100. In the periodic table it is in the group of actinides ( 7th period , f-block ) and is also one of the transuranic elements . Fermium is a radioactive metal, which, however, has not yet been represented as a metal due to the small quantities available. It was discovered in 1952 after the test of the first American hydrogen bomb and named in honor of Enrico Fermi , who, however, had nothing to do with the discovery of or research on fermium.
history


Fermium was found along with Einsteinium after testing the first American hydrogen bomb, Ivy Mike , on November 1, 1952 on the Eniwetok Atoll . The first samples were obtained on filter papers that were carried when flying through the explosion cloud. Larger amounts were later isolated from corals. For reasons of military secrecy, the results were initially not published.
A first investigation of the remains of the explosion had shown the formation of a new plutonium isotope 244 Pu, which could only have resulted from the uptake of six neutrons by a uranium- 238 core and two subsequent β-decays .
At the time, it was believed that the absorption of neutrons by a heavy nucleus was a rare occurrence. However, the identification of 244 Pu led to the conclusion that uranium nuclei can capture many neutrons, resulting in new elements.
The formation was achieved through continued neutron capture : At the moment of detonation, the neutron flux density was so high that most of the - radioactive - atomic nuclei that had formed in the meantime had not yet decayed by the next neutron capture. With a very high neutron flux, the mass number increases sharply without the atomic number changing. Only then do the resulting unstable nuclides decay over many β-decays to stable or unstable nuclides with a high atomic number:
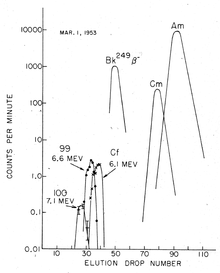
The discovery of fermium ( Z = 100) required more material as it was believed that the yield would be at least an order of magnitude lower than that of element 99. Therefore, contaminated corals from Eniwetok Atoll (where the test had taken place) were taken to the University of California Radiation Laboratory in Berkeley, California for processing and analysis. The dissolved actinide ions were separated in the presence of a citric acid / ammonium citrate buffer in a weakly acidic medium ( pH ≈ 3.5) with ion exchangers at an elevated temperature. About two months later, a new component was isolated, a high-energy α-emitter (7.1 MeV) with a half-life of about one day. With such a short half-life, it could only arise from the β-decay of an Einsteinium isotope, and so an isotope of element 100 had to be the new one: it was quickly identified as 255 Fm (t ½ = 20.07 hours).
In September 1953, there was no telling when the results of the teams in Berkeley , Argonne and Los Alamos would be published. It was decided to produce the new elements through bombardment experiments; At the same time, it was assured that these results would not fall under secrecy and could therefore be published. Einsteinium isotopes were produced shortly afterwards at the University of California Radiation Laboratory by bombarding uranium ( 238 U) with nitrogen ( 14 N). It was noted that there is research on this element that has so far been kept secret. Isotopes of the two newly discovered elements were generated by irradiating the plutonium isotope 239 Pu, and the results were published in five publications in quick succession. The final reactions from Californium are:
The Berkeley team was also concerned that another group of researchers might discover and publish the lighter isotopes of element 100 by ion bombardment before they could publish their classified research. Because at the end of 1953 and at the beginning of 1954 a working group from the Nobel Institute for Physics in Stockholm fired at uranium nuclei with oxygen nuclei; the isotope with the mass number 250 of the element 100 ( 250 μm) was formed. The unequivocal identification could be obtained from the characteristic energy of the α-particle emitted during the decay .
The Berkeley team has already published some results on the chemical properties of both elements. Finally, the results of the thermonuclear explosion were released in 1955 and then published.
Ultimately, the Berkeley team's priority was universally recognized, as their five publications preceded the Swedish publication and they could rely on the previously secret results of the 1952 thermonuclear explosion. This was associated with the privilege of naming the new elements. They decided to name them after famous scientists who had already died. It was quickly agreed to give the names in honor of Albert Einstein and Enrico Fermi , both of whom had recently died: “We suggest for the name for the element with the atomic number 99, einsteinium (symbol E) after Albert Einstein and for the name for the element with atomic number 100, fermium (symbol Fm), after Enrico Fermi. "The announcement for the two newly discovered elements Einsteinium and Fermium was made by Albert Ghiorso at the 1st Geneva Atomic Conference , which took place from 8 to Took place on August 20, 1955.
The element was later given the systematic name Unnilnilium for a time.
Isotopes
All 19 nuclides and 3 nuclear isomers known to date are radioactive and unstable. The known mass numbers range from 242 to 260. The isotope 257 Fm has by far the longest half-life with 100.5 days, so that there can be no more natural occurrences on earth. 253 Fm has a half-life of 3 days, 251 Fm of 5.3 hours, 252 Fm of 25.4 hours, 254 Fm of 3.2 hours, 255 Fm of 20.1 hours and 256 Fm of 2.6 hours. All the others have half-lives of 30 minutes to less than a millisecond.
If one takes out the decay of the longest-lived isotope 257 Fm, then first 253 Cf arises through α-decay , which in turn changes into 253 Es through β-decay . The further decay then leads via 249 Bk, 249 Cf, 245 Cm, 243 Am, 241 Pu, 241 Am to 237 Np, the beginning of the neptunium series (4 n + 1).
- The times given are half-lives.
 The decay from Fermium-257 to the Neptunium series .
The decay from Fermium-257 to the Neptunium series .
Fermium barrier
The fermium barrier is the fact that the fermium isotopes 258 Fm, 259 Fm and 260 Fm sometimes disintegrate spontaneously after just a fraction of a second (t ½ = 370 µs , 1.5 s or 4 ms). 257 Fm is an α-emitter and breaks down to 253 Cf. In addition, none of the previously known fermium isotopes shows β-decays, which prevents the formation of mendelevium by decay from fermium. These facts prevent practically any effort to produce elements with atomic numbers over 100 or mass numbers greater than 257 with the help of neutron radiation, for example with the help of a nuclear reactor. Fermium is thus the last element that can be produced by neutron capture. Any attempt to add more neutrons to a fermium nucleus leads to spontaneous fission.
Extraction
Fermium is produced by bombarding lighter actinides with neutrons in a nuclear reactor. The main source is the 85 MW high-flux isotope reactor at Oak Ridge National Laboratory in Tennessee, USA, which is set up for the production of transcurium elements (Z> 96).
In Oak Ridge larger amounts are to Curium been irradiated to decigram quantities of Californium , milligram quantities of berkelium and einsteinium and picogram to produce quantities of fermium. Nanogram and microgram quantities of fermium can be prepared for specific experiments. The amounts of fermium produced in thermonuclear explosions of 20 to 200 kilotons are believed to be on the order of a few milligrams, although it is mixed with a huge amount of explosion debris; 40 picograms of 257 Fm were isolated from 10 kilograms of the remains of the explosion from the Hutch test on July 16, 1969.
After irradiation, fermium must be separated from the other actinides and the lanthanoid fission products. This is usually achieved by ion exchange chromatography , the standard procedure runs with cation exchangers such as Dowex 50 or T EVA , elution is carried out with a solution of ammonium α-hydroxyisobutyrate . Smaller cations form more stable complexes with the methyl α-hydroxyisobutyrate anions, so they are preferentially eluted from the column. A rapid fractional crystallization method has also been described.
Although the most stable isotope of fermium is 257 Fm with a half-life of 100.5 days, most studies are based on 255 Fm (t ½ = 20.07 hours). This isotope can easily be isolated; it is a decay product of the 255 Es (t ½ = 39.8 days).
Small amounts of einsteinium and fermium were isolated and separated from plutonium , which was irradiated with neutrons. Four Einsteinium isotopes were found (with details of the half-lives measured at that time): 253 Es (α-emitters with t ½ = 20.03 days, as well as with a spontaneous fission half-life of 7 × 10 5 years); 254 m Es (β-emitters with t ½ = 38.5 hours), 254 Es (α-emitters with t ½ = ∼ 320 days) and 255 Es (β-emitters with t ½ = 24 days). Two fermium isotopes were found: 254 Fm (α-emitters with t ½ = 3.24 hours, as well as with a spontaneous fission half-life of 246 days) and 255 Fm (α-emitters with t ½ = 21.5 hours).
Bombarding uranium with five-fold ionized nitrogen and six-fold ionized oxygen atoms also produced Einsteinium and Fermium isotopes.
properties
In the periodic table , the fermium with atomic number 100 is in the series of actinides, its predecessor is the Einsteinium, the following element is the Mendelevium . Its analogue in the lanthanide series is erbium .
Physical Properties
The metal has not yet been shown, but measurements have been made on alloys with lanthanoids , and some calculations or predictions are available. The enthalpy of sublimation is directly related to the valence electron structure of the metal. The sublimation enthalpy of fermium was determined directly by measuring the partial pressure of the fermium using Fm- Sm and Fm / Es- Yb alloys in the temperature range from 642 to 905 K. They arrived at a value of 142 (13) kJ mol −1 . Since the sublimation enthalpy of fermium is similar to that of the divalent einsteinium, europium and ytterbium, it was concluded that fermium has a divalent metallic state. Comparisons with radii and melting points of europium, ytterbium and einsteinium metals resulted in estimated values of 198 pm and 1125 K for fermium.
The normal potential was estimated to be similar to the ytterbium Yb 3+ / Yb 2+ pair, i.e. about −1.15 V in relation to the standard hydrogen electrode , a value that agrees with theoretical calculations. On the basis of polarographic measurements, a normal potential of −2.37 V was determined for the Fm 2+ / Fm 0 pair. Fm 3+ can be reduced to Fm 2+ relatively easily , e.g. B. with samarium (II) chloride , precipitates together with the fermium.
Chemical properties
Until now, the chemistry of the fermium could only be investigated in solution with the help of tracer techniques; fixed connections were not established. Under normal conditions, fermium is in solution as an Fm 3+ ion, which has a hydration number of 16.9 and an acid constant of 1.6 · 10 −4 (pK s = 3.8). Fm 3+ forms complexes with a variety of organic ligands with hard donor atoms such as oxygen; and these complexes are usually more stable than those of the preceding actinides. It also forms anionic complexes with ligands such as chloride or nitrate; and these complexes also appear to be more stable than those of Einsteinium or Californium. It is assumed that the bond in the complexes of the higher actinides mostly has an ionic character: the Fm 3+ ion is, as expected, smaller than the previous An 3+ ions - due to the higher effective nuclear charge of fermium; and with that, fermium would likely form shorter and stronger metal-ligand bonds.
safety instructions
Classifications according to the CLP regulation are not available because they only include chemical hazard and play a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
use
Fermium - in the form of its compounds in solution - is primarily obtained in small quantities for study purposes. Compounds of the fermium have not yet been shown in solid form.
literature
- Robert J. Silva: Fermium, Mendelevium, Nobelium, and Lawrencium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1621-1651 ( doi: 10.1007 / 1-4020-3598-5_13 ).
- Glenn T. Seaborg (Ed.): Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100 , January 23, 1978; Report LBL-7701, April 1979.
- Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurans: Part A 1 II, pp. 19-20; Part A 2, p. 47; Part B 1, p. 84.
Web links
- Entry on fermium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Albert Ghiorso: Einsteinium and Fermium , Chemical & Engineering News, 2003.
Individual evidence
- ↑ The values of the atomic and physical properties (infobox) are, unless otherwise stated, taken from: Robert J. Silva: Fermium, Mendelevium, Nobelium, and Lawrencium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger ( Ed.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1621-1651.
- ↑ a b c d e Entry on fermium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e Entry on fermium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b c d e f g h Albert Ghiorso: Einsteinium and Fermium , Chemical & Engineering News, 2003.
- ↑ Albert Ghiorso , G. Bernard Rossi, Bernard G. Harvey, Stanley G. Thompson: Reactions of U 238 with Cyclotron-Produced Nitrogen Ions , in: Physical Review , 1954 , 93 (1), pp. 257-257 ( doi: 10.1103 / PhysRev.93.257 ).
- ↑ SG Thompson, A. Ghiorso, BG Harvey, GR Choppin: Transcurium Isotopes Produced in the Neutron Irradiation of Plutonium , in: Physical Review , 1954 , 93 (4), pp. 908-908 ( doi: 10.1103 / PhysRev.93.908 ) .
- ↑ BG Harvey, SG Thompson, A. Ghiorso, GR Choppin: Further Production of Transcurium Nuclides by Neutron Irradiation , in: Physical Review , 1954 , 93 (5), pp. 1129-1129 ( doi: 10.1103 / PhysRev.93.1129 ).
- ↑ MH Studier, PR Fields, H. Diamond, JF Mech, AM Friedman, PA Sellers, G. Pyle, CM Stevens, LB Magnusson, JR Huizenga: Elements 99 and 100 from Pile-Irradiated Plutonium , in: Physical Review , 1954 , 93 (6), pp. 1428-1428 ( doi: 10.1103 / PhysRev.93.1428 ).
- ↑ PR Fields, MH Studier, JF Mech, H. Diamond, AM Friedman, LB Magnusson, JR Huizenga: Additional Properties of Isotopes of Elements 99 and 100 , in: Physical Review , 1954 , 94 (1), pp. 209-210 ( doi: 10.1103 / PhysRev.94.209 ).
- ↑ GR Choppin, SG Thompson, A. Ghiorso, BG Harvey: Nuclear Properties of Some Isotopes of Californium, Elements 99 and 100 , in: Physical Review , 1954 , 94 (4), pp. 1080-1081 ( doi: 10.1103 / PhysRev .94.1080 ).
- ↑ Hugo Atterling, Wilhelm Forsling, Lennart W. Holm, Lars Melander, Björn Åström: Element 100 Produced by Means of Cyclotron-Accelerated Oxygen Ions , in: Physical Review , 1954 , 95 (2), pp. 585-586 ( doi: 10.1103 / PhysRev.95.585.2 ).
- ↑ a b G. T. Seaborg , SG Thompson, BG Harvey, GR Choppin: Chemical Properties of Elements 99 and 100 ; Abstract ; Machine script (July 23, 1954), Radiation Laboratory, University of California, Berkeley, UCRL-2591 (Rev.) (PDF; 1.5 MB).
- ^ A b S. G. Thompson, BG Harvey, GR Choppin, GT Seaborg: Chemical Properties of Elements 99 and 100 , in: J. Am. Chem. Soc. , 1954 , 76 (24), pp. 6229-6236 ( doi: 10.1021 / ja01653a004 ).
- ^ A b A. Ghiorso, SG Thompson, GH Higgins, GT Seaborg ( Radiation Laboratory and Department of Chemistry, University of California, Berkeley, California ), MH Studier, PR Fields, SM Fried, H. Diamond, JF Mech, GL Pyle , JR Huizenga, A. Hirsch, WM Manning ( Argonne National Laboratory, Lemont, Illinois ), CI Browne, HL Smith, RW Spence ( Los Alamos Scientific Laboratory, Los Alamos, New Mexico ): New Elements Einsteinium and Fermium, Atomic Numbers 99 and 100 , in: Physical Review , 1955 , 99 (3), pp. 1048-1049 ( doi: 10.1103 / PhysRev.99.1048 ; Maschinoscript (June 9, 1955), Lawrence Berkeley National Laboratory. Paper UCRL-3036 ).
- ^ PR Fields, MH Studier, H. Diamond, JF Mech, MG Inghram, GL Pyle, CM Stevens, S. Fried, WM Manning ( Argonne National Laboratory, Lemont, Illinois ); A. Ghiorso, SG Thompson, GH Higgins, GT Seaborg ( University of California, Berkeley, California ): Transplutonium Elements in Thermonuclear Test Debris , in: Physical Review , 1956 , 102 (1), pp. 180-182 ( doi: 10.1103 /PhysRev.102.180 ).
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics , 85th Edition, CRC Press, 2004, ISBN 978-0-8493-0485-9 , Section 4, pp. 4–10 ( limited preview in Google Book Search) . This is no longer mentioned in the 90th edition (pp. 4–12 to 4–13).
- ↑ G. Pfennig, H. Klewe-Nebenius, W. Seelmann-Eggebert (eds.): Karlsruher Nuklidkarte , 7th edition, 2006.
- ^ A b G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties , in: Nuclear Physics A , 729, 2003, pp. 3–128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ a b c d e f Robert J. Silva: Fermium, Mendelevium, Nobelium, and Lawrencium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Ed.): The Chemistry of the Actinide and Transactinide Elements , 3 3rd edition, Springer, Dordrecht 2006, Volume 3, pp. 1621-1651.
- ^ High Flux Isotope Reactor , Oak Ridge National Laboratory; Retrieved September 23, 2010.
- ↑ a b C. E. Porter, FD Riley, Jr., RD Vandergrift, LK Felker: Fermium Purification Using Teva ™ Resin Extraction Chromatography , in: Sep. Sci. Technol. , 1997 , 32 (1-4), pp. 83-92 ( doi: 10.1080 / 01496399708003188 ).
- ↑ M. Sewtz, H. Backe, A. Dretzke, G. Kube, W. Lauth, P. Schwamb, K. Eberhardt, C. Grüning, P. Thörle, N. Trautmann, P. Kunz, J. Lassen, G . Passler, CZ Dong, S. Fritzsche, RG Haire: First Observation of Atomic Levels for the Element Fermium ( Z = 100) , in: Phys. Rev. Lett. , 2003 , 90 (16), p. 163002 ( doi: 10.1103 / PhysRevLett.90.163002 ).
- ↑ RW Hoff, EK Hulet: Engineering with Nuclear Explosives , 1970 , 2 , pp. 1283-1294.
- ^ GR Choppin, BG Harvey, SG Thompson: A new eluant for the separation of the actinide elements , in: J. Inorg. Nucl. Chem. , 1956 , 2 (1), pp. 66-68 ( doi: 10.1016 / 0022-1902 (56) 80105-X ).
- ↑ NB Mikheev, AN Kamenskaya, NA Konovalova, IA Rumer, SA Kulyukhin: High-speed method for the separation of fermium from actinides and lanthanides , in: Radiokhimiya , 1983 , 25 (2), pp. 158-161.
- ↑ M. Jones, RP Schuman, JP Butler, G. Cowper, TA Eastwood, HG Jackson: Isotopes of Einsteinium and Fermium Produced by Neutron Irradiation of Plutonium , in: Physical Review , 1956 , 102 (1), pp. 203-207 ( doi: 10.1103 / PhysRev.102.203 ).
- ↑ LI Guseva, KV Filippova, Yu. B. Gerlit, VA Druin, BF Myasoedov, NI Tarantin: Experiments on the Production of Einsteinium and Fermium with a Cyclotron , in: Journal of Nuclear Energy , 1954 , 3 (4), pp. 341–346 (translated in November 1956) ( doi: 10.1016 / 0891-3919 (56) 90064-X ).
- ↑ NB Mikheev, VI Spitsyn, AN Kamenskaya, NA Konovalova, IA Rumer, LN Auerman, AM Podorozhnyi: Determination of oxidation potential of the pair Fm 2+ / Fm 3+ , in: Inorg. Nucl. Chem. Lett. , 1977 , 13 (12), pp. 651-656 ( doi: 10.1016 / 0020-1650 (77) 80074-3 ).
- ^ LJ Nugent, in: MTP Int. Rev. Sci .: Inorg. Chem., Ser. One , 1975 , 7 , pp. 195-219.
- ↑ K. Samhoun, F. David, RL Hahn, GD O'Kelley, JR Tarrant, DE Hobart: Electrochemical study of mendelevium in aqueous solution: No evidence for monovalent ions , in: J. Inorg. Nucl. Chem. , 1979 , 41 (12), pp. 1749-1754 ( doi: 10.1016 / 0022-1902 (79) 80117-7 ).
- ↑ Jaromír Malý: The amalgamation behavior of heavy elements 1. Observation of anomalous preference in formation of amalgams of californium, einsteinium, and fermium , in: Inorg. Nucl. Chem. Lett. , 1967 , 3 (9), pp. 373-381 ( doi: 10.1016 / 0020-1650 (67) 80046-1 ).
- ↑ NB Mikheev, VI Spitsyn, AN Kamenskaya, BA Gvozdec, VA Druin, IA Rumer, RA Dyachkova, NA Rozenkevitch, LN Auerman: Reduction of fermium to divalent state in chloride aqueous ethanolic solutions , in: Inorg. Nucl. Chem. Lett. , 1972 , 8 (11), pp. 929-936 ( doi: 10.1016 / 0020-1650 (72) 80202-2 ).
- ↑ EK Hulet, RW Lougheed, PA Baisden, JH Landrum, JF Wild, RF Lundqvist: Non-observance of monovalent Md , in: J. Inorg. Nucl. Chem. , 1979 , 41 (12), pp. 1743-1747 ( doi: 10.1016 / 0022-1902 (79) 80116-5 ).
- ^ Robert Lundqvist, EK Hulet, TA Baisden: Electromigration Method in Tracer Studies of Complex Chemistry. II. Hydrated Radii and Hydration Numbers of Trivalent Actinides , in: Acta Chem. Scand., Ser. A , 1981 , 35 , pp. 653-661 ( doi: 10.3891 / acta.chem.scand.35a-0653 ).
- ↑ H. Hussonnois, S. Hubert, L. Aubin, R. Guillaumont, G. Boussieres: Radiochem. Radioanal. Lett. , 1972 , 10 , pp. 231-238.
![\ mathrm {^ {238} _ {\ 92} U \ {\ xrightarrow [{- 2 \ \ beta ^ {-}}] {+ \ 6 \ (n, \ gamma)}} \ _ {\ 94} ^ {244} Pooh}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c4b60ac4bf0d4ebf96b6a71fefa86f13dd5784e0)
![\ mathrm {^ {238} _ {\ 92} U \ \ xrightarrow [-8 \ \ beta ^ -] {+ \ 15, \ 16, \ 17 \ (n, \ gamma)} \ ^ {253, \ 254 , \ 255} _ {\ \ \ \ \ \ \ \ \ \ \ 100} Fm}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c2b40de4e6caa3766ff5f34a85f77466ab79326f)
![\ mathrm {^ {252} _ {\ 98} Cf \ {\ xrightarrow {(n, \ gamma)}} \ _ {\ 98} ^ {253} Cf \ {\ xrightarrow [{17.81 \ d}] {\ beta ^ {-}}} \ _ {\ 99} ^ {253} It \ {\ xrightarrow {(n, \ gamma)}} \ _ {\ 99} ^ {254} It \ {\ xrightarrow [{ }] {\ beta ^ {-}}} \ _ {100} ^ {254} Fm}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2dcf5875c42aad904ed8c8a397de8fc552cf5f71)


![\ mathrm {^ {257} _ {100} Fm \ \ xrightarrow [100.5 \ d] {\ alpha} \ ^ {253} _ {\ 98} Cf \ \ xrightarrow [17.81 \ d] {\ beta ^ -} \ ^ {253} _ {\ 99} Es \ \ xrightarrow [20.47 \ d] {\ alpha} \ ^ {249} _ {\ 97} Bk \ rightarrow \ ... \ rightarrow \ ^ { 237} _ {\ 93} Np}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0976bf22416778cd427bb5b7cf5b80a38f25162b)