Lanthanoids
|
Position in the periodic table
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lanthanoide [ lantanoˈiːdə ] ("lanthanum-like"; Greek : ending -ειδἠς ( -eides ) "similar") is a group name for similar elements . Attributed to it are the lanthanum and 14 in the periodic table following elements cerium , praseodymium , neodymium , promethium , samarium , europium , gadolinium , terbium , dysprosium , holmium , erbium , thulium , ytterbium and lutetium . In the sense of the term, lanthanum does not belong to the group of lanthanum-like. Here, however, the IUPAC nomenclature follows practical use. The use of the old name lanthanide is still allowed. All lanthanides are metals and are also known as elements of the lanthanum series. They are part of the group of rare earth metals .
|
57 La |
58 Ce |
59 Pr |
60 Nd |
61 pm |
62 Sm |
63 Eu |
64 Gd |
65 p |
66 Dy |
67 Ho |
68 he |
69 Tm |
70 yb |
71 Lu |
Occurrence
The lanthanoids are also known as rare earth metals. This name is confusing because the elements of this group, with the exception of the unstable promethium, are by no means as rare as it is suggested. For example, cerium is more common in nature than the elements arsenic or lead . They are involved in the structure of the earth's crust to a mass fraction of 0.02%. There are a total of 15 elements of the 6th period , of which the 14 elements following lanthanum can be understood as a subgroup of the 3rd subgroup .
Due to their chemical similarity, the lanthanides are usually associated in nature. Since the separation of the individual lanthanides is difficult and their chemical properties are very similar, these elements are often grouped under the (unofficial) chemical symbol Ln (not to be confused with La for lanthanum). Many of them can be obtained from monazite (also called secondary deposits - monazite sands). The most common and economically most important lanthanoid-bearing minerals are:
- Monazite CePO 4
- Xenotime YPO 4
- Bastnaesite LNCO 3 F
- Parisit CALN 2 (CO 3 ) 3 F 2
- Allanite CaLn (Al, Fe 2+ ) 3 Si 3 O 11 OH
- Synchysite CALN (CO 3 ) 2 F
- Ankylite (Ce) SrCe (CO 3 ) 2 (OH) · H 2 O
- Ankylite- (La) Sr (La, Ce) [OH | (CO 3 ) 2 ] · H 2 O
- Cerianite CeO 2
- In the formulas, Ln denotes all elements from lanthanum to lutetium and the very similar yttrium (Y).
In almost all minerals there is an accumulation of either the light (Ce) or the heavy lanthanoids (Y behaves in terms of mineral chemistry like a heavy lanthanoid). For example, monazite contains predominantly Ce and La, while the content of the following lanthanides decreases with the atomic number (therefore the monazite formula is always given as CePO 4 ). In Xenotim one finds exactly the opposite case (hence also YPO 4 ). This mostly very effective fractionation is caused by the lanthanide contraction and the crystal lattice sites available, which vary in size from mineral to mineral. Other mineral groups can also incorporate high proportions of lanthanides into their structure (e.g. zircon , garnet ). Furthermore, the lanthanides occur on the moon in the form of the so-called KREEP ores .
properties
Physical Properties
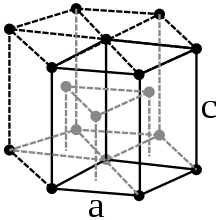
The lanthanoids are silvery, shiny, relatively soft and reactive metals. Almost all of them have the closest packing of spheres , which is typical for metals . The hardness increases with increasing atomic number.
Like actinides, lanthanoids belong to the inner transition elements or f-block elements , since the f orbitals in these rows are not completely filled with electrons.
The promethium isotopes are all unstable, i.e. radioactive.
Chemical properties
Due to the similar structure of the valence shell , the lanthanoids behave chemically like the elements of the 3rd group of the periodic table scandium and yttrium and together with these form the group of rare earths . They oxidize quickly in air and become dull. They react more or less quickly with water to form hydrogen .
Starting with Cer, the 4f orbital is gradually filled up. In the case of lutetium, it is completely occupied with 14 electrons. Since the 4f orbitals lie deep inside the atoms , they have little influence on the chemical behavior in contrast to the d orbitals of the other subgroup elements. The lanthanide elements are therefore relatively similar in terms of their chemical properties. They are so similar that when ytter earth was discovered in 1794, they were even thought to be the oxide of one and the same element. The same applies to the numerous components of the cerite earth. What they have in common is the oxidation number +3. In addition, the oxidation numbers +2 and +4 occur with some elements.
A discontinuity in the course of the ionic radii between gadolinium and terbium is called a gadolinium break in lanthanides. This explains why, despite the similarity of the lanthanoids, the chemical behavior of the elements changes after the gadolinium. The chemical behavior can easily be influenced on the Gadolinium Break. Traces of americium are sufficient for a terbium complex to adopt the structure type of the lighter lanthanoids.
Colors of the lanthanoid ions in an aqueous solution
| Oxidation number |
57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +2 |
Sm 2+ blood red |
Eu 2+ colorless |
Tm 2+ purple red |
Yb 2+ yellow green |
|||||||||||
| +3 |
La 3+ colorless |
Ce 3+ colorless |
Pr 3+ yellow-green |
Nd 3+ violet |
Pm 3+ purple pink |
Sm 3+ deep yellow |
Eu 3+ colorless |
Gd 3+ colorless |
Tb 3+ colorless |
Dy 3+ yellow green |
Ho 3+ yellow |
He 3+ deep pink |
Tm 3+ pale green |
Yb 3+ colorless |
Lu 3+ colorless |
| +4 |
Ce 4+ orange-yellow |
Pr 4+ yellow |
Nd 4+ blue-violet |
Tb 4+ red-brown |
Dy 4+ orange-yellow |
||||||||||
Lanthanide contraction
Due to the lanthanide contraction, the atomic radius decreases almost steadily within the range from cerium (183 pm) to lutetium (172 pm) (exceptions are europium and ytterbium). This is due to the fact that the elements that - based on the atomic number - are in front of the lanthanides have already filled the 6s and 5p shells with electrons, but not the 4f shell. The lanthanoids now fill the 4f shell with electrons. In a simplified representation of the atom as consisting of spatially separated electron shells, an electron shell that is spatially closer to the nucleus is now filled with charge carriers. Incidentally, the nucleus naturally fills with the same number of protons as electrons are added to the 4f shell. The resulting stronger attraction between electrons and protons reduces the atomic radius while the atomic number increases.
This effect is actually not exceptional, as the radius always decreases when a bowl is filled within a period. However, this property has some consequences:
- Due to the decreasing size, separation by means of ion exchangers is easily possible.
- In the holmium, the radius of Ln 3+ is so small that it almost corresponds to that of Y 3+ ; therefore yttrium is usually found together with the "heavy earths"
- Within a group, the transition elements in the 2nd and 3rd positions have very similar properties.
use
There are numerous examples of the uses of the lanthanides:
- Cerium: is the main component in Auermetall , which is used as a “flint” in lighters, as an oxide in self-cleaning ovens and as a catalyst in cracking .
- Praseodymium: is in yellow colored glass, e.g. B. in welding goggles.
- Neodymium: mainly used for strong magnets. Is also part of welding safety glasses and is used in solid lasers instead of rubies.
- Promethium: serves as a heat source in (unmanned) satellites and space probes.
- Samarium: is used as a permanent magnet, e.g. B. in headphones.
- Europium: serves as a neutron absorber in nuclear power reactors, but also as an activator of the red fluorescent substances in television tubes.
- Gadolinium: can also be found in the television tube as an activator of the green phosphors.
- Terbium: is used as a laser material.
- Dysprosium: is found as a neutron absorber in nuclear power reactors.
- Holmium: only found in alloys. In general, the lanthanoids are often found in alloys, they make steel easier to process.
- Erbium: found in photographic filters.
- Thulium: also serves as a neutron absorber in nuclear power plants.
- Ytterbium: produces X-rays without electricity, e.g. B. in portable X-ray machines.
- Lutetium: is a catalyst in cracking and polymerizing.
See also
Remarks
- ↑ Before the introduction of ion exchangers, different valence levels could only be used for cerium (trivalent and tetravalent) or europium (bivalent and trivalent); the other elements had to be recrystallized hundreds of times to separate them .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1928-1947.
- James E. Huheey: Inorganische Chemie , 1st edition, de Gruyter, Berlin 1988, ISBN 3-11-008163-6 , pp. 873-900.
- Norman N. Greenwood, Alan Earnshaw: Chemistry of the Elements , 1st Edition, VCH, Weinheim 1988, ISBN 3-527-26169-9 , pp. 1571-1600.
- dtv-Atlas zur Chemie 1981 , Part 1, pp. 216-221.
Web links
- Literature by and about lanthanoids in the catalog of the German National Library
- W. Thum: The discovery of the rare earth metals . A summary for the lesson prepared from a didactic point of view. chemie-master.de
Individual evidence
- ↑ Wolfgang Liebscher, Ekkehard Fluck: The systematic nomenclature of inorganic chemistry . Springer-Verlag, Berlin 1999, ISBN 3-540-63097-X .
- ^ Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 .
- ^ AM Mariano: Economic geology of rare earth minerals . In: BR Lipin, GA McKay (Ed.): Reviews in Mineralogy , Vol. 21 - Geochemistry and mineralogy of rare earth elements (1989). Published by the Mineralogical Society of America, ISBN 0-939950-25-1 , pp. 309-337.
- ↑ Georg Steinhauser: Structural Chemistry - A Touch of Nothing. In: News from chemistry. 66, 2018, p. 118, doi : 10.1002 / nadc.20184067855 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1937.
- ↑ dtv atlas on chemistry . 1981 , part 1, p. 220.
- ↑ a b Lanthanide lecture script from the University of Bayreuth.