Ruthenium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Ruthenium, Ru, 44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 8 , 5 , d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-18-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-127-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.297 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.02 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 101.07 (2) & | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 130 (178) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 146 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Kr ] 4 d 7 5 s 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.36050 (5) eV ≈ 710.18 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 16.76 (6) eV ≈ 1 617 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 28.47 eV ≈ 2 747 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 45.0 (1.7 eV) ≈ 4 342 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 59.0 (1.9) eV ≈ 5 693 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 12.37 g / cm 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 6.6 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2607 K (2334 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 4423 K (4150 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 8.17 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 619 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 25.7 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 1.4 Pa at 2523 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 5970 m · s −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 14.1 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 120 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3, 4 , 6, 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.2 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ruthenium (from Latin Ruthenia " Ruthenia ", " Russia ") is a chemical element with the element symbol Ru and the atomic number 44. It is one of the transition metals , in the periodic table it is in the 5th period and group 8 (formerly part of the 8th subgroup ) or iron group . It is a silvery white, hard and brittle platinum metal .
Ruthenium was discovered in Siberian platinum ores in 1844 by the German-Baltic chemist Karl Ernst Claus . It is very rare and only used in small amounts. The main areas of application for the metal are in the electronics industry for perpendicular recording , a data storage method for hard drives , and as a catalyst in various chemical processes such as hydrogenation , methanation or ammonia synthesis . Some ruthenium compounds, e.g. B. the Grubbs catalysts , also play a role in chemical syntheses.
Ruthenium has no known biological functions, however some complexes of the metal are being investigated for their effects as anti- cancer agents .
history
After the four platinum metals palladium , rhodium , iridium and osmium were discovered in platinum ores by William Hyde Wollaston and Smithson Tennant in quick succession between 1803 and 1804 , other chemists also tried to isolate previously unknown elements from such ores.
The Polish chemist Jędrzej Śniadecki first reported in 1808 that the year before he had discovered a new element in rare South American platinum ores. He named it after the recently discovered asteroid Vesta Vestium. However, after this discovery could not be verified by other chemists, the discovery was rejected again.
After the discovery of large platinum ore deposits in the Urals in 1819, Jöns Jakob Berzelius in Stockholm and Gottfried Osann in Tartu began to investigate them. In 1828 Osann initially received an unknown white oxide, whose properties did not match any other oxide, and after reduction an unknown golden yellow metal. He named this ruthenium after the country of origin of the ore Russia . However, after Berzelius could not confirm this discovery, Osann repeated his work, but could not repeat the isolation of ruthenium and thereupon withdrew his discovery.
The German-Baltic chemist Karl Ernst Claus tried since 1841 at the University of Kazan to repeat Osann's experiments and to extract unknown elements from platinum ores. He finally succeeded in doing this in 1844 when he was able to extract six grams of an unknown light gray metal. Like Osann, he called the new element ruthenium. Just like Osann, Claus asked Berzelius to review the experiments and confirm the new element. Since he was able to confirm the results in 1845, Claus has been considered the discoverer of ruthenium ever since.
Occurrence
Ruthenium is one of the rarest non-radioactive elements on earth. Its abundance is about 1 ppb by mass in the earth's crust , while it is contained in the earth's shell (crust up to 16 km depth) with a mass fraction of 20 ppb. The frequency is comparable to that of rhodium, iridium or rhenium . It is usually with other platinum metals associated , the proportion of ruthenium is in the main platinum metal deposit, the South African Bushveld Complex , between eight and twelve percent.
Like other platinum metals, it occurs naturally in nature and is therefore recognized by the IMA as a mineral with system no. 1.AF.05 (class: elements , department: metals and intermetallic compounds , subdivision: platinum group elements ) recognized.
Its type locality , in which the mineral was first found in 1974 by Y. Urashima, T. Wakabayashi, T. Masaki and Y. Terasaki, is located on the Uryū River on the Japanese island of Hokkaidō . In addition to this, another 21 sites of elemental ruthenium are known. These include the Nizhny Tagil and the Miass River in Russia, the Yuba River in California and the Bushveld Complex in South Africa.
In addition to elemental ruthenium, various ruthenium-containing minerals are also known. The 13 currently known (status: 2010) are alloys with other platinum metals such as rutheniridosmin , sulfides such as laurite (RuS 2 ) or arsenides such as ruthen arsenite (Ru, Ni) As.
Extraction and presentation
Due to the similarities and low reactivity of the platinum metals, separating these elements is complicated. There are several ways to isolate ruthenium. If an ore contains a high concentration of ruthenium, the separation of the ruthenium is best done first and is achieved by distillation . For this purpose, a solution containing trivalent or hexavalent ruthenium is mixed with oxidizing agents such as chlorine , chlorates or potassium permanganate . This oxidizes the ruthenium to the volatile ruthenium (VIII) oxide . This can be captured in dilute hydrochloric acid and reduced to water-soluble chlororuthenate complexes. The reason for this procedure is the dangers that exist due to the formation of ruthenium (VIII) oxide during the separation. The reaction of ruthenium (VIII) oxide with ammonium salts can produce explosive nitrogen-chlorine compounds.
If the starting material only contains small amounts of ruthenium, the remaining platinum metals are separated off first. There are different methods for this purpose for the different metals, in particular extraction with suitable solvents or the precipitation of the sparingly soluble salts. Ultimately, the dissolved ruthenium remains. The solution is freed from any ammonium present, the ruthenium is oxidized to ruthenium (VIII) oxide and separated off by distillation.
To obtain metallic ruthenium, it is either precipitated as ammonium hexachlororuthenate or as ruthenium (IV) oxide and reduced at 800 ° C. in a hydrogen atmosphere .
In addition to platinum ores, the anode sludge that occurs during nickel production is an important raw material for the extraction of ruthenium and the other platinum metals.
Another occurrence of ruthenium are spent fuel elements , since platinum metals are also formed during nuclear fission . One ton of these fuel elements contains over two kilograms of ruthenium, but also more valuable platinum metals such as rhodium or palladium. This ruthenium from spent fuel elements contains almost 4% radioactive 106 Ru (soft beta emitter, half-life approx. 1 year), which decays to 106 Rh. The rhodium decays immediately (half-life 30 s) with emission of gamma rays . Use of ruthenium from nuclear reactors is therefore not foreseeable under current circumstances.
The world production of ruthenium is in the range of approx. 20 t per year (as of 2008).
properties
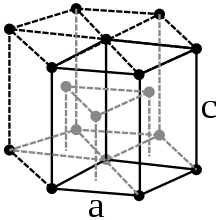
Physical Properties
Ruthenium is a silvery white, hard and brittle metal. With a density of 12.37 g / cm 3 , it is the second lightest platinum metal after palladium . Ruthenium melts at 2606 K and boils at around 4423 K. Below 0.49 K, the element becomes a superconductor .
Just like osmium, ruthenium crystallizes in a hexagonal- tight packing of spheres in the space group P 6 3 / mmc (space group no.194 ) with the lattice parameters a = 270.6 pm and c = 428.1 pm as well as two formula units per unit cell . Occasionally, four different polymorphic forms of ruthenium are specified, into which the metal changes when heated to temperatures of 1308, 1473 and 1770 K. However, these are based on calorimetric measurements from 1931, which could not be confirmed in the subsequent period. Therefore, it is likely that the element has only one modification up to the melting point. A metastable tetragonal modification was found in very thin films on a molybdenum surface. This shows ferromagnetic properties at room temperature .
Chemical properties
Within the iron group, ruthenium has similar properties to osmium, while it differs significantly from those of iron . Like other platinum metals, it is an inert noble metal in contrast to iron . It only reacts with the oxygen in the air at temperatures above 700 ° C and forms ruthenium (VIII) oxide . It also differs from osmium, which forms traces of the corresponding osmium (VIII) oxide when it comes into contact with oxygen at room temperature . Ruthenium also only reacts with fluorine and chlorine in the heat and forms ruthenium (VI) fluoride or ruthenium (III) chloride .
The metal does not dissolve in acids such as B. hydrofluoric acid , sulfuric acid , nitric acid or aqua regia . On the other hand, it is attacked slowly by aqueous chlorine and bromine solutions, quickly by cyanide solutions and mercury (II) chloride . Strong oxidizing agents such as potassium hydroxide - potassium nitrate - or sodium hydroxide - sodium peroxide - melts oxidize ruthenium quickly.
Isotopes
A total of 33 isotopes and a further six core isomers of ruthenium between 87 Ru and 120 Ru are known. Of these, seven are stable and also occur in nature. The most common is the isotope 102 Ru with a share of 31.6% of the natural isotope composition. Four isotopes, 104 Ru, 101 Ru, 100 Ru and 99 Ru are similarly common with proportions between 12 and 19%. The rarest of the stable isotopes are 96 Ru and 98 Ru with proportions of 5.52 and 1.88%, respectively. Of the unstable isotopes, only 97 Ru (2.9 days), 103 Ru (39.26 days) and 106 Ru (373.59 days) have half-lives of a few days; those of the others are in the range from milliseconds ( 103m1 Ru: 1.69 ms) to hours ( 105 Ru: 4.44 h).
Ruthenium isotopes, especially 101 Ru, 102 Ru and 104 Ru, are formed during nuclear fission and are therefore present in spent fuel elements . One ton of uranium used in nuclear fission contains around 1.9 kilograms of ruthenium as a fission product. During reprocessing, this can be separated from the mixture dissolved in nitric acid by oxidation to volatile ruthenium (VIII) oxide. Since this ruthenium also contains a portion of the radioactive isotope 106 Ru, which is relatively long-lived with a half-life of 373 days , it cannot be used directly for other purposes.
use
Ruthenium is only used to a limited extent. Most of the metal is used in the electronics industry. Registered since 2006 mainly playing perpendicular recording a role, a method of storing data on disks , in which a thin layer of ruthenium, the storage layer of a cobalt - chromium - platinum - alloy of a soft magnetic separates underclass. The reason why ruthenium is used lies in its hexagonal crystal structure , which has a lattice constant similar to that of the storage layer alloy used. Thin ruthenium layers are used in electrical contacts such as slip rings or reed relays . Compared to other metals that can be used, such as cobalt-hardened gold, they are harder and therefore more resistant to abrasion.
Like other platinum metals, ruthenium has a catalytic effect . Thus, it can be about the hydrogenation of aromatics , acids and ketones are used. Ruthenium also has a catalytic effect in methanation , the production of methane from hydrogen and carbon monoxide or carbon dioxide . So far, however, ruthenium has found only minor uses for methanation; mostly nickel catalysts are used. The lower temperatures required for methanation with ruthenium could be of interest for long-term space missions, as the carbon dioxide exhaled by the astronauts could be converted and the oxygen cycle closed.
Analogous to iron and osmium , ruthenium also catalyzes the synthesis of ammonia from nitrogen and hydrogen . It has a higher catalyst activity than iron and thus enables a higher yield at lower pressures. The use of the metal is primarily limited by its price. A ruthenium catalyst, which is supported on a carbon matrix and improved by barium and cesium as promoters , has been in industrial use since 1998 in two KBR production facilities in Trinidad . Since the slow methanation of the carbon carrier interferes with the process, research is being carried out on carbon-free ruthenium catalysts for ammonia synthesis.
In small quantities, ruthenium is used in alloys of palladium or platinum to increase the hardness. Alloys containing ruthenium are used, among other things, for nibs in fountain pens or for dental fillings . Small amounts of ruthenium (0.1%) make titanium alloys more corrosion-resistant, which is important for applications in the chemical industry or oil production. It is a possible alternative to palladium. Also in superalloys on nickel-based , which for turbine blades are used, ruthenium may be an alloy component, it causes an increased phase stability here.
A large part of the ruthenium is not used in the form of the metal, but as a compound, primarily as ruthenium (IV) oxide , which is used, among other things, as a material for resistors and electrodes , for example for the coating of titanium anodes in chlor-alkali electrolysis .
The beta emitter 106 Ru is used for radiation therapy of the choroidal melanoma .
Biological importance
Like other platinum metals, ruthenium has no biological significance and does not normally occur in the body. Various ruthenium complexes have pharmacological potential. Various applications as an active ingredient are being researched. Some compounds are already being tested in clinical studies . Most importantly, this is the effect as a cytostatic agent , so as a means of therapy of cancer . Here, ruthenium complexes are possible alternatives to cisplatin or carboplatin . In addition to the tumor-inhibiting effect that the compounds of several platinum metals have, this is based primarily on three properties of ruthenium complexes:
- They have a slow ligand exchange so that the complex can reach the right place in the body without reacting with water or other molecules,
- several possible oxidation states (+2, +3, +4) under physiological conditions, as well
- bears a great resemblance to iron , so they can replace it in proteins like transferrin .
Since trivalent ruthenium is relatively inactive, while divalent ruthenium shows a strong tumor-inhibiting effect, it should be possible to reduce trivalent ruthenium in a tumor to bivalent one and thus activate it. This would allow a more selective effect than with other cytostatics. So far, no drug based on ruthenium has been approved .
In addition to the use in antineoplastic chemotherapy , applications of ruthenium compounds as immunosuppressive agents , antibiotics and antimicrobial substances , for example for combating malaria or Chagas disease , are being investigated.
Precautions
As a metal, ruthenium is non-toxic. In contrast to osmium, the poisonous and highly volatile tetraoxide is not formed through reaction with oxygen at room temperature, but only through reaction with strong oxidizing agents. In powder form, ruthenium is flammable; in the event of a fire, it must not be extinguished with water, but only with extinguishing powder or metal fire extinguishers.
links
Ruthenium forms compounds in the oxidation states −2 to +8, the most stable and most common are +3 and +4. Together with osmium and xenon, it is one of the elements in which the highest oxidation level +8 can be chemically achieved.
Oxygen compounds
Ruthenium forms three binary oxides with oxygen , ruthenium (VIII) oxide , ruthenium (VI) oxide and ruthenium (IV) oxide . In addition, ruthenium (III) oxide , but only as a hydrate, and various ruthenates , including orange ruthenate (VI), salts whose anion is a ruthenium-oxygen compound , are known. Like osmium (VIII) oxide , ruthenium (VIII) oxide is a yellow, volatile and poisonous compound which is obtained by reacting ruthenium or its compounds with strong oxidizing agents and which is used as a strong oxidizing agent and for the separation of ruthenium from other platinum metals is important. While ruthenium (VI) oxide is only known in the gas phase, ruthenium (IV) oxide is a stable salt that crystallizes in the rutile structure and is used, among other things, in resistors and for coating electrodes.
In contrast to osmium, no octavalent ruthenate is known from ruthenium; in aqueous solutions, when reacting with strong oxidizing agents, a yellow-green perruthenate, corresponding to permanganate , is formed. This also acts as an oxidizing agent, but is milder and therefore more selective than ruthenium (VIII) oxide or osmium (VIII) oxide. Primary alcohols are not oxidized to carboxylic acids by perruthenates , but only to aldehydes . It is often used in organic syntheses in the form of tetrapropylammonium perruthenate (TPAP). It is reduced to tetravalent ruthenium.
Complexes

Many are of the ruthenium complex compounds with both inorganic and organic ligands known. These can exist in very different oxidation states from −2 to +8. In intermediate stages, such as +2, +3, and +4, nonclassical complexes have also been synthesized that contain metal clusters with ruthenium-ruthenium bonds.
Some ruthenium complexes have found use as catalysts in various organic syntheses. For example, ruthenium is the central metal in the complexes of the Grubbs' catalysts , which are among the most important catalysts for olefin metathesis . Another complex that is important in organic synthesis is the Noyori catalyst , a ruthenium-chlorine- BINAP complex that enables efficient asymmetric hydrogenation of β-keto esters.

Ruthenium complexes are able to catalyze polymerizations . In addition to ring-opening polymerization (ROMP) based on metathesis , living free radical polymerizations can also be made possible by ruthenium complexes. An example of this is the polymerization of methyl methacrylate with RuCl 2 (PPh 3 ) 3 as a catalyst.
One of the best-known ruthenium complexes is the amine complex ruthenium red , which is used in histology as a staining agent and as a redox indicator and for examining textile fibers. Another example of a ruthenium complex is (1,5-cyclooctadiene) (1,3,5-cyclooctatriene) ruthenium , which was first synthesized in 1963 by Ernst Otto Fischer .
Other ruthenium compounds
With the halogens fluorine , chlorine , bromine and iodine , ruthenium forms a number of compounds. The trivalent ruthenium halides are the most stable; these are also known from all halogens. Only the fluorides up to ruthenium (VI) fluoride and the unstable ruthenium (IV) chloride are known in higher oxidation states . The most important of these compounds is ruthenium (III) chloride , which is a starting material for the synthesis of many other ruthenium compounds.
The category: ruthenium compounds offers an overview of ruthenium compounds .
Release of 106 Ru south of the Urals in 2017
In October 2017, increased atmospheric 106 Ru concentrations in the order of 10 mBq / m 3 were measured in several European countries , which, however, were far below normal air activities. The analysis of the air currents suggested a source south of the Urals in Russia . At that time, the SOX-Borexino experiment in Gran Sasso, which is supposed to find “sterile” neutrinos, required a compact, extremely active radiation source. These were ordered from a reprocessing plant in Mayak . The highly enriched cerium required for this is only supplied by freshly burned nuclear fuel rods. The proportion of 103 Ru in the samples suggests that the spent fuel elements were processed only two years after the end of the power plant operation in Mayak. Such highly radioactive fuel elements are difficult to process. In this case, ruthenium is separated off anyway. It escapes as a gaseous ruthenium tetroxide. High air concentrations of 106 Ru were measured 30 kilometers away from Mayak in the municipality of Argayash . The activity released was estimated to be 100–300 terabecquerel , a risky amount for the local population. In February 2018, the French newspaper Le Figaro described the task of French and Italian researchers. Mayak announced in December 2017 that the Cer-144 ordered could not be delivered because the process did not reach the required level. This presumption of causes is also explained in a study published in 2019.
literature
- Entry to ruthenium. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- Hermann Renner u. a .: Platinum Group Metals and Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2001, doi: 10.1002 / 14356007.a21_075 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
Web links
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (ruthenium) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on ruthenium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on ruthenium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b c Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ a b Data sheet ruthenium from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ A b John Emsley: Nature's Building Blocks. An A – Z Guide to the Elements. Oxford University Press, Oxford 2001, ISBN 0-19-850341-5 , pp. 368-369.
- ↑ G. Osann: Continuation of the investigation of the platinum from the Urals. In: Poggendorff's annals of physics and chemistry . 14, 1828, pp. 329-257 ( digitized on Gallica ).
- ↑ G. Osann: Correction, concerning my investigation of the Urals platinum. In: Poggendorff's annals of physics and chemistry. 15, 1829, p. 158 ( digitized on Gallica ).
- ↑ Helvi Hödrejärv: Gottfried Wilhelm Osann and ruthenium. In: Proceedings of the Estonian Academy of Sciences, Chemistry. 53, No. 3, 2004, pp. 125–144 ( limited preview in Google book search).
- ↑ UN Pitchkov: The Discovery of ruthenium. (PDF; 689 kB) In: Platinum Metals Review. 40, 4, 1996, pp. 181-188.
- ↑ uniterra.de
- ↑ a b periodensystem-online.de
- ↑ periodensystem.info
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. CRC, Boca Raton 2009, ISBN 978-1-4200-9084-0 (Section 14, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea).
- ↑ a b c Hermann Renner u. a .: Platinum Group Metals and Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2001, doi: 10.1002 / 14356007.a21_075 .
- ↑ IMA / CNMNC List of Mineral Names - Ruthenium ( Memento of the original from March 20, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (English, PDF 1.8 MB, p. 247).
- ↑ Jolyon Ralph, Ida Chau: Ruthenium . In: mindat.org. Retrieved April 12, 2010.
- ↑ Webmineral - Mineral Species sorted by the element Ru (Ruthenium) (English).
- ^ Zdenek Kolarik, Edouard V. Renard: Potential Applications of Fission Platinoids in Industry. (PDF; 379 kB) In: Platinum Metals Review. 49, 2005, pp. 79-90.
- ↑ a b United States Geological Survey (Ed.): 2008 Minerals Yearbook - Platinum-Group Metals (PDF; 64 kB). 2007.
- ^ Mark Winter: Ruthenium: physical properties . In: Webelements.com. Retrieved April 28, 2010.
- ↑ K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica. 30, 1974, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ↑ a b Joseph A. Rard: Chemistry and thermodynamics of ruthenium and some of its inorganic compounds and aqueous species. In: Chemical Reviews. 85, No. 1, 1985, pp. 1-39, doi: 10.1021 / cr00065a001 .
- ↑ P. Quarterman, Congli Sun, Javier Garcia-Barriocanal, Mahendra DC, Yang Lv, Sasikanth Manipatruni, Dmitri E. Nikonov, Ian A. Young, Paul M. Voyles, Jian-Ping Wang: Demonstration of Ru as the 4th ferromagnetic element at room temperature. In: Nature Communications. 9, 2018, doi: 10.1038 / s41467-018-04512-1 .
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729, 2003, pp. 3-128. doi: 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ^ RP Bush: Recovery of Platinum Group Metals from High Level Radioactive Waste. (PDF; 494 kB) In: Platinum Metals Review. 35, No. 4, 1991, pp. 202-208.
- ↑ Martin Volkmer: Basic knowledge of nuclear energy. Nuclear Energy Information Circle , Bonn 1996, ISBN 3-925986-09-X , p. 80.
- ↑ JZ Shi u. a .: Influence of dual-Ru intermediate layers on magnetic properties and recording performance of CoCrPt-SiO 2 perpendicular recording media. In: Applied Physics Letters. 87, 2005, pp. 222503-222506, doi: 10.1063 / 1.2137447 .
- ^ Paul C. Hydes: Electrodeposited Ruthenium as an Electrical Contact Material. (PDF; 452 kB) In: Platinum Metals Review. 24, No. 2, 1980, pp. 50-55.
- ↑ a b c Entry on ruthenium. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ^ Yvonne Traa, Jens Weitkamp: Kinetics of the methanation of carbon dioxide on ruthenium on titanium dioxide. In: Chemical Engineer Technology. 70, No. 11, 1998, pp. 1428-1430, doi: 10.1002 / cite.330701115 .
- ↑ Heinz Hiller u. a .: Gas Production. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2001, doi: 10.1002 / 14356007.a12_169.pub2 .
- ↑ Max Appl: Ammonia. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2006, doi: 10.1002 / 14356007.a02_143.pub2 .
- ↑ Hubert Bielawa, Olaf Hinrichsen, Alexander Birkner, Martin Muhler: The next-generation ammonia catalyst: barium-promoted ruthenium on oxidic carriers. In: Angewandte Chemie. 113, No. 6, 2001, pp. 1093-1096, doi : 10.1002 / 1521-3757 (20010316) 113: 6 <1093 :: AID-ANGE10930> 3.0.CO; 2-3 .
- ^ Karl Eichner, Heinrich F. Kappert: Dental materials and their processing. 8th edition. Thieme, Stuttgart 2005, ISBN 3-13-127148-5 , p. 93.
- ↑ RW protection: Ruthenium Enhanced Titanium Alloys. (PDF; 474 kB) In: Platinum Metals Reviews. 40, No. 2, 1996, pp. 54-61.
- ↑ Yutaka Koizumi et al. a .: Development of a next-generation Ni-base single crystal superalloy. (PDF; 313 kB) In: Proceedings of the International Gas Turbine Congress 2003 Tokyo. 2003.
- ↑ Holger Voigt: Malignant melanoma. Springer-Verlag, 2013, ISBN 978-3-642-70460-4 , p. 56 ( limited preview in the Google book search).
- ↑ Choroidal Melanoma - Medical Information. In: radioonkologie.uniklinikum-leipzig.de. April 15, 2015, accessed October 9, 2017 .
- ^ A b Claire S. Allardyce, Paul J. Dyson: Ruthenium in Medicine: Current Clinical Uses and Future Prospects. (PDF; 612 kB) In: Platinum Metals Review. 45, No. 2, 2001, pp. 62-69.
- ↑ Emmanuel S. Antonarakis, Ashkan Emadi: Ruthenium-based chemotherapeutics: are they ready for prime time? In: Cancer Chemotherapy and Pharmacology . 66, No. 1, 2010, pp. 1-9, doi: 10.1007 / s00280-010-1293-1 .
- ^ Entry on ruthenium in the GESTIS substance database of the IFA , accessed on April 27, 2008(JavaScript required) .
- ↑ Steven V. Ley, Joanne Norman, William P. Griffith, Stephen P. Marsden: Tetrapropylammonium Perruthenate, Pr 4 N + RuO 4 - , TPAP: A Catalytic Oxidant for Organic Synthesis. In: Synthesis. 7, 1994, pp. 639-666, doi: 10.1055 / s-1994-25538 .
- ^ Holleman-Wiberg, Textbook of Inorganic Chemistry, 101st edition, de Gruyter Verlag 1995 ISBN 3-11-012641-9 .
- ↑ Christoph Elschenbroich : Organometallchemie. 6th edition. Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 632-633, 642.
- ^ Ruthenium in Living Radical Polymerization. (PDF; 114 kB) In: Platinum Metals Review. 43, No. 3, 1999, p. 102.
- ↑ Entry on ruthenium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ Ernst Otto Fischer, Jörn Müller: Metal π complexes of ruthenium and osmium with 6- and 8-membered cyclic oligoolefins. In: Chemical Reports . 96, 1963, pp. 3217-3222, doi: 10.1002 / cber.19630961217 .
- ↑ Origin in Russia? - Ruthenium-106 values attract attention. n-tv.de, November 21, 2017, accessed November 30, 2017.
- ↑ Neutrino experiment was behind the nuclear accident . Spektrum-Verlag, July 29, 2019, accessed on July 30, 2019
- ↑ Une commande franco-Italienne à l'origine de la pollution au ruthénium 106? , Le Figaro, February 2, 2018
- ↑ Airborne concentrations and chemical considerations of radioactive ruthenium from an undeclared major nuclear release in 2017 . PNAS, July 26, 2019, accessed July 28, 2019.
- ↑ Puzzle about radioactive cloud solved . science.orf.at, July 27, 2019, accessed on July 28, 2019.



