catalysis
Catalysis (from ancient Greek κατάλυσις katálysis , German 'dissolution' ) describes the change in the kinetics of a chemical reaction by means of a catalyst with the aim of getting it going in the first place, accelerating it or directing selectivity in a favored direction. In the living cell, enzymes that catalyze biochemical processes play a fundamental role in the metabolism, from digestion to the reproduction and transcription of genetic information . In the environmental sector, both naturally occurring catalytic processes such as the formation of smog are of great importance, as is the catalytic reduction of pollutants in the automotive and power plant sectors. New systems for energy conversion and storage such as the fuel cell are based on catalytic processes.
The added value through catalysis in the chemical industry is of considerable economic importance, since over 80% of all chemical products are manufactured with the help of catalytic processes. By optimizing them, the energy and resource expenditure can be significantly reduced. Worldwide sales for catalysts in 2007 were around 16 billion US dollars, of which over 90% were generated with catalysts for heterogeneously catalyzed processes.
history
First discoveries
The first catalytic technical processes used by humans are alcohol fermentation from sugar, used by the Sumerians in Mesopotamia as early as 6000 BC, as well as the production of acetic acid from alcohol with the help of catalytically active enzymes. After these early beginnings, it was not until the 18th and early 19th centuries that a whole series of new catalytic reactions were discovered. In 1781 , Antoine-Augustin Parmentier discovered the splitting of starch into sugar under acid catalysis. Only one year later, Carl Wilhelm Scheele discovered the acid-catalyzed esterification of alcohols and acids to esters in 1782 and shortly afterwards Joseph Priestley in 1783 discovered the decomposition of ethanol into ethylene and water on alumina .
As the first process for the technical production of a basic chemical, Desormes and Clement developed the lead-chamber process for the production of sulfuric acid in 1806 , in which nitrogen oxides catalyze the oxidation of sulfur dioxide. Berthollet discovered the decomposition of ammonia into nitrogen and hydrogen on iron catalysts, and in 1818 Thénard discovered the decomposition of hydrogen peroxide into silver , silver oxide and manganese dioxide . The ignition of hydrogen on platinum discovered by Döbereiner in 1823 led to the development of the Döbereiner lighter , which was manufactured in relatively large numbers and was used until the middle of the 19th century. In a letter to Johann Wolfgang von Goethe , his superior, Döbereiner wrote about his discovery: Gracious Minister of State! I allow myself, Ew. Excellency of a discovery to give news ... that the purely metallic powdery platinum has the most remarkable property of determining the hydrogen gas by mere touch and without any involvement of external potencies that it combines with oxygen gas to form water, one of which is until the platinum burns out increased sum of heat is excited.
Works by Berzelius and Ostwald

In 1835, Berzelius recognized the commonality in the above reactions that, in addition to the starting materials and products, another substance was always necessary in the reaction that was apparently not consumed. He coined the term catalysis in analogy to analysis : ... I will call it the catalytic power of the body, and the decomposition by the same catalysis. The catalytic force really seems to consist in the fact that bodies are able to awaken their mere presence, and not through their kinship, the kinships slumbering at this temperature, so that according to them in a composite body the elements change into such other proportions by which a greater electrical-chemical neutralization is produced.

Wilhelm Ostwald found a modern definition of catalysis in 1894. It reads: Catalysis is the acceleration of a slow chemical process through the presence of a foreign substance . Specified later on: A catalyst is a substance that increases the speed of a chemical reaction without being consumed itself and without changing the final position of the thermodynamic equilibrium of this reaction. In recognition of his work on catalysis as well as for his fundamental studies of chemical equilibrium conditions and reaction rates, Ostwald was awarded the Nobel Prize in Chemistry in 1909 .
Catalysis in food production
In addition to the further development of sulfuric acid production in the contact process as a large-scale catalysis process, heterogeneous catalysis was also used in the field of food production. In 1901 , Wilhelm Normann discovered fat hardening through the catalytic hydrogenation of oleic acid to stearic acid with hydrogen on finely divided nickel and thus the basis for large-scale industrial margarine production. In 1909 the process was used on a large scale and in a plant in Warrington in England 100 tons of whale oil were processed into edible fats per week according to Normann's process .
The French chemist Victor Henri was working in the field of enzyme catalysis as early as 1903. He investigated the breakdown of sucrose into glucose and fructose with the help of the enzyme sucrose . The continuation of his work by the German biochemist Leonor Michaelis and the Canadian physician Maud Menten succeeded in formulating the Michaelis-Menten theory in 1913 , the cornerstone of enzyme kinetics that is still valid today . The potential of enzyme catalysis for the resource-saving production of fine chemicals, pharmaceuticals, vitamins or detergents is far from being exhausted to this day, over 100 years after the fundamentals were discovered.
Large-scale industrial processes
In the early 20th century, a number of processes began to be developed that are still among the most important in the chemical industry today. In 1910 Haber , Bosch and Mittasch developed the synthesis of ammonia from the elements nitrogen and hydrogen on iron contacts, the Haber-Bosch process . Wilhelm Ostwald developed the Ostwald process of ammonia oxidation on platinum networks to nitric acid , which made the previously scarce nitrate fertilizer available on a large scale.
In 1913, the first Wacker process for the production of acetaldehyde from acetylene and water on mercury catalysts was discovered. By the fluid catalytic cracking of silica / alumina catalysts gasoline was higher from petroleum fractions accessed later by the hydrocracking of nickel catalysts.
In 1923, Matthias Pier developed a high-pressure catalytic process at BASF for the synthesis of methanol from synthesis gas on zinc oxide-chromium oxide catalysts. This made an important basic chemical of industrial organic chemistry available that was used in many other processes. Fischer and Tropsch invented the Fischer-Tropsch process , with which carbon monoxide and hydrogen obtained from coal for the first time were converted to paraffins and olefins over iron-cobalt catalysts from 1925 . The process is gaining in importance again today in order to obtain not only fuels but also chemical raw materials, such as olefins, from resources other than petroleum. At around the same time Walter Reppe discovered the homogeneous catalytic reaction of acetylenes with various reactants under nickel complex catalysis to form a wide range of products, the so-called Reppe chemistry .
In 1938 Otto Roelen discovered hydroformylation, the production of aldehydes from olefins, carbon monoxide and hydrogen over cobalt catalysts, which he developed further into an industrial process. Hydroformylation is considered to be the first large-scale application of homogeneous transition metal catalysts. The original Roelens process has been further developed many times. Today, the Ruhrchemie-Rhone-Poulenc process, which works with water-soluble homogeneous rhodium catalysts for easier separation of the catalyst, is the state of the art in hydroformylation technology.
Other catalytic processes have also been developed in the field of refinery technology. By catalytic reforming of niedrigoktanigen alkanes over platinum tin - or platinum rhenium / alumina contacts developed high-octane, aromatics and isoalkane-rich gasolines. The process still provides several million liters of high-octane gasoline per day.
With the low-pressure process developed by Karl Ziegler at the Max Planck Institute for Coal Research , in which ethylene and propylene are converted to polyolefins on titanium / aluminum catalysts, the basis for the petrochemical industrial mass production of polymers was laid, which heralded the age of plastics . Ziegler and Giulio Natta were awarded the Nobel Prize in Chemistry for this work . At the MPI in Mülheim an der Ruhr, the fundamental work of Günther Wilke , who discovered the production of 1,5-cyclooctadiene from 1,3-butadiene on nickel catalysts, as well as the work of Wilhelm Keim on the SHOP process, was also carried out .
More Nobel Prizes
The understanding of enzyme catalysis and its stereochemistry was expanded through the work of Cornforth , who was awarded the Nobel Prize in Chemistry for this. In addition to the development of catalytic processes for basic and intermediate products, numerous processes for the production of fine chemicals have also been developed over the years.
The work of William S. Knowles and Ryoji Noyori “for their work on chirally catalyzing hydrogenation reactions” and the epoxidation named after Barry Sharpless were awarded the Nobel Prize.
Also in the 1970s, Richard F. Heck discovered the cross-coupling catalyzed with homogeneous palladium complexes, which allows the direct olefination of aryl halides. Another Nobel Prize in the field of catalysis was awarded to Chauvin , Schrock, and Grubbs in 2005 for the discovery of alkene metathesis of olefins over ruthenium catalysts . In 2007, Gerhard Ertl was honored for his studies of chemical processes on solid surfaces that allow extensive insights into the elementary steps of heterogeneously catalyzed reactions.
Economical meaning
The economic importance of catalysis is enormous. A large part of the world's population is fed by food produced with mineral fertilizers. In 2002, the world market for nitrogenous fertilizers was around 144 million tonnes of ammonia using the Haber-Bosch process. About 1% of the global energy expenditure is necessary for this. The savings potential by increasing the efficiency of the catalytic processes is therefore great.
Catalysis plays a key role in the area of more effective material and thermal utilization of fossil and renewable raw materials as well as in the environmental area. Catalytic processes are used in refineries to produce low-sulfur and high-octane fuels. UOP, one of the leading manufacturers of catalytic reforming systems, states the capacity of the CCR systems installed worldwide according to the UOP patent with over 600 million liters of gasoline per day.
In environmental technology, car exhaust catalysis, the catalytic reduction of nitrogen oxides using the Denox process and diesel catalytic converters make a decisive contribution to keeping the air clean. The use of catalytic burners in building services is intended to reduce the formation of nitrogen oxides due to the lower combustion temperature that this makes possible.
Acrolein, hydrocyanic acid and ammonia
In the food production sector, enzyme-catalytic and acid / base-catalyzed processes are traditionally used. Amino acids such as D, L-methionine are increasingly being produced from raw materials obtained by heterogeneous catalysis such as acrolein , hydrocyanic acid and ammonia . In the field of active ingredient production, processes for producing enantiomerically pure products are particularly sought after. In addition to the homogeneous catalytic processes, biocatalytic processes are particularly advantageous here.
In the area of renewable raw materials, the use of catalytic processes is currently limited mainly to the base-catalyzed transesterification of triglycerides with methanol to form biodiesel . The installed production capacity of biodiesel plants in Germany was 4.85 million tons per year in 2008, of which 2.8 million tons or around 8% of the total diesel demand were sold.
Starch, cellulose and sugar
The renewable raw materials starch, cellulose and sugar were mainly used in enzyme-catalytic fermentation processes. In 2007 a total of around 2.1 million tons of renewable raw materials were used in the chemical industry.
Fossil raw materials
In the field of fossil raw materials, intensive research is being carried out into new catalytic processes. The partial oxidation of methane, by far the largest economically usable source of hydrocarbons according to current estimates, to synthesis gas or the direct heterogeneous catalytic or enzyme catalytic oxidation with methane monooxygenase to methanol are the focus of current research.
Energetic basics

A simple chemical reaction A + B → AB can, for example , be influenced by a catalyst cat. As follows , where A-KAT corresponds to the transition state:
Catalysts increase the speed of chemical reactions by several orders of magnitude. However, it is not possible to use catalysts to carry out reactions in which the total energy of the end products is higher than that of the starting materials. Such reactions are thermodynamically forbidden even with catalysts : catalysis is therefore a kinetic and not a thermodynamic phenomenon. As with any spontaneous reaction, the free enthalpy of reaction ( ) must be negative. A catalyst does not change the chemical equilibrium of a reaction, only the speed with which it occurs. The catalytic effectiveness is based solely on the ability to lower the activation energy of a chemical reaction. That is the amount of energy that must first be overcome in order to set the reaction in motion. During the reaction, the reactant is changed increasingly, it adopts an energetically unfavorable transition state . The activation energy is the amount of energy required to force the reactant into the transition state. This is where the effect of the catalyst comes in, which increases the probability of the reactant adopting the transition state due to its spatial structure and its charge ratio. Thus, less energy is required to bring the reactant into this state. The Arrhenius equation gives the effect of a low activation energy with otherwise identical reaction conditions on the reaction rate constant:
With
- Pre-exponential factor or frequency factor , according to the impact theory corresponds to the product of the impact number Z and the orientation factor P,
- Activation energy (unit: J / mol)
- = 8.314 J / (K mol) general gas constant
- absolute (thermodynamic) temperature (unit: K)
- Reaction rate constant
The catalysis is therefore to be regarded as a purely kinetic effect.
Classification
The catalysis is classified according to various criteria such as the phases involved or the type of catalysts used. Depending on the phase in which the catalyst and substrate are present, a distinction is made between homogeneous (catalyst and substrate are in the same phase) and heterogeneous catalysis (catalyst and substrate are in different phases). If a catalyst or substrate changes phase during the reaction, the process is called phase transfer catalysis .
Depending on the type of catalyst used, there are further classification criteria such as transition metal catalysis, acid-base catalysis or biocatalysis. If the products of a catalytic reaction have special stereochemical properties, this is called enantioselective catalysis . If a substance produced during a reaction has a catalytic effect on the generation reaction, this process is called autocatalysis . Depending on the type of catalyzed reaction, one speaks, for example, of oxidation or hydrogenation catalysts .
If reactions are catalyzed with the help of organic molecules that do not contain any metals but only consist of light main group elements, this is called organocatalysis . In organocatalysis, the catalyst initially reacts with the substrate to form a covalent bond. A well-known example is enantioselective proline catalysis. As a secondary amine, proline forms an enamine with a carbonyl compound , which is in tautomeric equilibrium with its iminium ion. In both forms, the resulting complex can undergo a number of reactions, such as nucleophilic aldol additions or Knoevenagel reactions .
In the last step, the product is released through hydrolysis and the proline recovered as a catalyst.
Heterogeneous catalysis
In heterogeneous catalysis, the catalyst is often in solid form (so-called contact) and the substrate reacts from the gas or liquid phase. Heterogeneous catalysis is the basis of many chemical and petrochemical processes. Catalysts are often solid bases and acids such as zeolites, metal oxides or heterogenized homogeneous catalysts such as supported enzymes. In addition to the chemistry of the catalytic reaction, transport processes are often rate-determining steps in heterogeneously catalyzed reactions. A great advantage of heterogeneous catalysts is their easy separation from the reaction products.
The optimization of catalytic processes requires a multidisciplinary approach in which, in addition to the chemistry of the catalyst and the process, aspects of fluid dynamics , reactor design and new findings in solid-state and surface chemistry and analytics must be taken into account.
Homogeneous catalysis
In homogeneous catalysis, the catalyst and substrate can either be in the gas phase, such as NO 2 and SO 2 in the lead chamber process, or in the liquid phase. The selectivity is often higher than in heterogeneously catalyzed reactions, but it is often difficult to separate them from the resulting product mixture.
If the activity of the homogeneous catalyst used is very high and if it goes through sufficient catalytic cycles, it is not necessary to separate the catalyst. In such cases, for example in the Ziegler-Natta process, the catalyst remains in the product in a very small amount.
Depending on the type of catalyst used, a distinction is made between the following types of homogeneous catalysis:
- Brönsted acid / base catalysis,
- Lewis acid / base catalysis, or electrophilic / nucleophilic catalysis,
- Redox catalysis,
- Complex catalysis,
- organometallic complex catalysis.
Phase transfer catalysis
If a catalyst enables contact between two reactants that are in different phases (usually aqueous and organic phase), this is called phase transfer catalysis. The catalyst of such a reaction enables the reactants to pass through the phase boundary.
To enable crown ether , the solution of alkali metal ions into organic solvents; the anions, for example MnO 4 -, are dragged into the organic phase as a crown ether / alkali anion pair. Crown ethers with 15 and 18 carbon atoms in the ring have proven particularly useful. Good enantiomeric excesses can be obtained by using chiral crown ethers.
Quaternary ammonium compounds , phosphonium or arsonium ions with lipophilic alkyl residues improve the extraction. The lipophilicity increases with an increasing carbon content in the alkyl radical. The rate of reaction of phase transfer catalysis often increases linearly with the concentration of the catalyst.
Biocatalysis
Metabolic processes in living things are catalyzed by enzymes . These reactions are generally characterized by extremely high efficiency and selectivity and take place at mild temperatures and in an aqueous medium. Reactive species that would react with water are shielded by hydrophobic "pockets". Many biocatalysts are proteins or contain protein components. According to their function, the enzymes are divided into six classes as oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases.
Some biocatalytic processes are among the oldest known chemical processes known to man, such as beer brewing . Biocatalytic processes using bacteria, yeasts or fungi, especially in the production of wine, beer, cheese and other foods, have been known for a long time. Biocatalytic processes are also increasingly used in the pharmaceutical industry . Economically important biocatalytic processes are the production of vitamin C , the production of aspartame , the saccharification of starch to glucose syrup and the antibiotics -production by enzymatic cleavage of penicillin G .
catalyst
A catalyst is the substance that causes the catalysis described above. Catalysts are used for stationary and mobile environmental protection, in the chemical industry and in refinery technology. Sales of catalytic converters for automobiles amounted to approximately 5.2 billion US dollars in 2008. The automobile catalytic converter thus had the largest share of the market for heterogeneous catalytic converters. Catalysts for stationary environmental protection applications, such as DENOX catalysts, generated sales of around 1 billion dollars worldwide. About 30% of the market is for catalysts for refinery processes such as platforming and catcracking , another 30% is used in a variety of processes in the chemical industry.
The efficiency or activity of a catalytic converter is expressed by the number of turns , also known as the turn-over number , or by the number of turns per unit of time, the turn-over frequency . Another essential property of a catalyst is its selectivity, that is, its ability to accelerate only the desired reactions but avoid the generation of undesired by-products.
In addition to the actual catalyst, a catalytic cycle often requires a cocatalyst that has a positive effect on the activity or selectivity of a catalyst. A well-known example is the function of methylaluminoxane as a co-catalyst in olefin polymerization and hydriodic acid in the Monsanto process .
Production of heterogeneous catalysts
The effectiveness of heterogeneous catalysts depends essentially on the number of simultaneous possible contacts between the catalyst and the substrates, for which the largest possible catalyst surface is often important. Heterogeneous catalysts can be in the form of non-porous full contacts or metal networks, as in the Ostwald process, with low specific surface areas or as porous solids with a high specific surface area. Full contacts can be produced, for example, by precipitation or co-precipitation of metal salts by alkali carbonates or hydroxides. After precipitation, these are washed, dried, calcined and, if necessary, activated by reducing them to metal. The catalysis of oxidation reactions often requires full contacts, which have the active component only in the outer area of the catalyst body and allow rapid diffusion of the reactants from and into the main gas flow, the rate-determining step in these reactions.
Heterogeneous catalysts are made in many different ways. A known process is extrusion for the production of catalytic converters for the automobile exhaust catalytic converter, which is then coated with a washcoat. A porous contact consists either of solid material such as aluminum oxide or of a carrier material that has been impregnated or coated with a catalytically active component and optionally activators.
An important aspect of the porous contacts is the distribution of the pore sizes. The pores in the catalyst grain increase the specific surface area considerably. This can be several hundred square meters per gram. According to the IUPAC definition , there are three pore size ranges: micropores with a diameter of less than 2 nm, mesopores in the range from 2 to 50 nm and macropores that are larger than 50 nm. For the design of the catalyst it is important that the pore size distribution is matched to the diffusion and reaction rate of the reaction.
After a carrier has been impregnated with a metal salt solution , the metal is dried , calcined and activated , for example . As a result of the specific conditions in these steps, special metal profiles can optionally be set on a carrier pellet , which must be tailored to the reaction kinetics and the diffusion characteristics of the reactants in the catalyzed reaction.
In heterogeneous catalysis, support materials carry the finely divided, catalytically active metal clusters and, due to their properties, can serve as cocatalysts, or as ligands they have an influence on the catalytic activity of the dispersed metal. In the case of microporous substances such as zeolites, ion exchange has proven itself.
With special structures, for example in the case of zeolites, support materials can influence the selectivity of a reaction. Examples of support materials are cordierite , carbon black , silica gel , zeolites or metal oxides such as titanium dioxide and aluminum oxide .
Production of homogeneous transition metal catalysts
The production of homogeneous transition metal catalysts is often done using the methods of organometallic chemistry. Many transition metal catalysts are sensitive to air and moisture. The ligand design is of particular importance for the yield and selectivity of the homogeneously catalyzed reaction. The electronic and steric properties of the ligand can control the reaction and, for example, transfer steric information to the reactant system.
Catalyst deactivation and regeneration
The mechanisms of catalyst deactivation are diverse. In transition metal catalysis , for example, the reduction of the metal-ligand catalysts used to the metal is observed.
The type of deactivation can be roughly divided into mechanical, for example through abrasion or disintegration, thermal such as for example sintering, physical such as coking or the physical blockage of active centers, and chemical deactivation through the formation of inactive metal components such as sulfides.
In heterogeneous catalysis, coking, sintering of the active surface or the disintegration of the catalyst due to mechanical abrasion, for example in fluid bed processes, are known in refinery processes. Aging processes can reduce the catalytically active surface or clog pores, for example in zeolites.
The regeneration processes include, for example, the burning off of coke from contacts that are used in cracking processes or catalytic reforming, or oxychlorination to restore acidic centers. If the catalytic converter is deactivated to such an extent that regeneration no longer makes sense, the catalytic converter is discharged from the process. In the case of noble metal catalysts , the supports are melted if necessary and the noble metal is recovered through smelting and electrochemical processes.
Deactivation through phase changes is often irreversible. This is observed, for example, with zinc / aluminum oxide catalysts for the synthesis of methanol that have been exposed to excessively high temperatures. The catalyst is deactivated by the formation of a spinel phase and cannot be regenerated.
Catalytic Mechanisms
Mechanisms in Heterogeneous Catalysis
In addition to the chemical reaction, the transport processes can be the rate-determining steps in heterogeneous catalysis. A total of seven steps can be distinguished. The first is the diffusion of the reactants from the gas stream into the catalyst. The second step is pore diffusion, followed by the adsorption of the reactants on the active site. After the chemical reaction, the products leave the contact through desorption, pore diffusion and diffusion into the main gas flow.
The chemical process after adsorption can take place in different ways. In monomolecular reactions, for example, the educt breaks down on the surface of the catalyst. In the case of bimolecular reactions, three mechanisms are conceivable, which depend on whether and how the reactants are adsorbed.
Langmuir-Hinshelwood mechanism
The Langmuir - Hinshelwood mechanism consists of the steps of adsorption, surface diffusion, surface reaction and desorption. First, both starting materials A and B must be adsorbed from the gas phase on the catalyst surface :
The adsorbed species diffuse on the surface of the catalyst and then react to form product C:
In the last step, product C desorbs :
The Langmuir-Hinshelwood mechanism has been demonstrated for the oxidation of CO on platinum catalysts, the methanol synthesis on zinc oxide catalysts and the oxidation of ethylene to acetaldehyde on palladium catalysts, among other things.
Eley-Rideal mechanism
In the Eley-Rideal mechanism , which was proposed by DD Eley and Eric Rideal in 1938 , reactant A initially adsorbs on the catalyst surface:
The adsorbed educt then reacts with another educt B from the gas phase to form product C:
In the last step, product C desorbs :
The Eley-Rideal mechanism has been demonstrated for the oxidation of ammonia on platinum catalysts and the selective hydrogenation of acetylene on iron catalysts.
Mars van Krevelen mechanism
Educt A is first adsorbed from the gas phase on the catalyst surface:
This is followed by the oxidation of educt A with the lattice oxygen present:
Product AO desorbs and an oxygen vacancy arises in the crystal lattice:
After desorption, the vacancies are filled up again by reoxidation with oxygen:
The oxidative dehydrogenation of propane to propene proceeds according to a Mars-van-Krevelen mechanism on vanadium-containing metal oxide catalysts.
Mechanisms in homogeneous catalysis
Well-known forms of homogeneous catalysis are acid-base catalysis, transition metal catalysis and organocatalysis. The mechanisms of organocatalysis are diverse. More recently, the use of chiral amino acids such as proline in enantioselective syntheses has been investigated. Compared to transition metal catalysis, organocatalysis hardly needs to be carried out with the exclusion of air or water; the catalysts are easily obtained from natural substances and can be used in different solvents.
Acid-base catalysis
A well-known example of an acid-catalyzed reaction is the above-mentioned esterification of carboxylic acids with alcohols or the reversal of this reaction with saponification . A distinction is made between two types, specific and general acid or base catalysis.
In the case of specific acid catalysis in a solvent S, the reaction rate is proportional to the concentration of the protonated solvent molecule HS + .
The acid itself, AH, only takes part in the reaction in the form that it shifts the equilibrium between the solvent and the acid to the protonated form of the solvent SH + :
In a buffered aqueous solution, the rate of reaction of the reactant R depends only on the pH of the system, but not on the concentration of the acid involved.
This type of chemical kinetics is observed when the reactant R 1 is in rapid equilibrium with its conjugate acid R 1 H + , which then slowly further reacts with R 2 to form the reaction products, for example in the case of aldol condensation .
In general acid catalysis, all species that can provide a proton contribute to increasing the reaction rate, with strong acids being more effective.
Proton transfer is the rate-limiting step in general acid catalysis, for example in azo coupling .
Transition metal catalysis
The first step in homogeneous transition metal catalysis is the formation of the active species from the pre-catalyst. On the actual catalyst, the oxidative addition of a substrate takes place in the second step, changing the oxidation state of the catalyst metal. In a further step, another substrate complexes the catalytically active species. A typical reaction in the following step is the insertion of this substrate into the previously formed substrate-catalyst bond of the oxidatively added substrate. The catalytic cycle is closed by rearrangement and subsequent reductive elimination of the product formed, releasing the original catalyst.
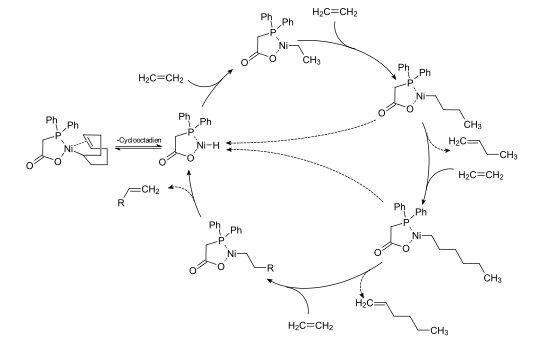
In the SHOP process, the catalytically active species is formed by splitting off the cyclooctadiene ligand from the nickel-diphenylphosphinoacetic acid-COD complex. By inserting ethene into the nickel-hydrogen bond, a nickel-alkyl complex is created, into whose nickel-carbon bond further ethene units can be inserted. By reductive elimination of the α-olefin, the nickel-hydrogen-diphenylphosphinoacetic acid complex is released again, which again represents the starting point for further catalytic cycles.
Other examples of homogeneously catalyzed processes are hydrocyanation , hydroformylation or olefin metathesis .
Nobel Prizes in Catalysis
| year | person | Reason for awarding the prize |
|---|---|---|
| 1909 | Wilhelm Ostwald | "In recognition of his work on catalysis as well as for his fundamental studies of chemical equilibrium conditions and reaction rates" |
| 1912 | Paul Sabatier | "For his method of hydrogenating organic compounds in the presence of finely divided metals, whereby the progress of organic chemistry has been promoted to a large extent in recent years" |
| 1918 | Fritz Haber | "For the synthesis of ammonia from its elements" (Haber-Bosch process) |
| 1932 | Irving Langmuir | "For his discoveries and research in the field of surface chemistry" |
| 1946 | James Batcheller Sumner | "For his discovery of the crystallizability of enzymes" |
| 1963 |
Karl Ziegler , Giulio Natta |
"For their discoveries in the field of chemistry and the technology of high polymers" ( Ziegler-Natta process ) |
| 1975 | John W. Cornforth | "For his work on the stereochemistry of enzyme-catalysis reactions" |
| 2001 |
William S. Knowles , Ryoji Noyori |
"For her work on chirally catalyzing hydrogenation reactions" |
| Barry Sharpless | "For his work on chiral catalyzing oxidation reactions" ( Sharpless epoxidation ) | |
| 2005 |
Yves Chauvin , Robert Grubbs , Richard R. Schrock |
"For the development of the metathesis method in organic synthesis" |
| 2007 | Gerhard Ertl | "For his studies of chemical processes on solid surfaces" |
| 2010 |
Richard F. Heck , Ei-ichi Negishi , Akira Suzuki |
"For palladium -catalyzed cross-couplings in organic synthesis " |
Organizations
Various associations in Europe act as an interface for exchange between industry and universities at national and international level. The organizations support the promotion of research and teaching and serve as interest groups in the field of catalysis.
In Germany, the German Catalysis Society (GECATS) is active at the European level, the European Federation of Catalysis Societies (EFCATS) represents scientific organizations in the field of catalysis in 25 countries. At the international level, the International Association of Catalysis Societies (IACS) has organized the International Congress on Catalysis (ICC) since 1956. After the last ISS Congress in Munich in 2012, the next one will take place in Beijing in July 2016. In North America, the North American Catalysis Society (NACS) represents the interests of catalysis.
Trade journals (selection)
The current state of research in the field of catalysis is published in a large number of journals and specialist journals. These include the Catalysis Letters , which are edited by Norbert Kruse and Gábor A. Somorjai and which publish articles in the field of homogeneous, heterogeneous and enzymatic catalysis.
The Catalysis Reviews emphasize the interdisciplinary aspect of catalysis and publish articles in various areas such as reactor design, computer simulation and advances in analytical processes.
Applied Catalysis magazine will be published in an A and B issue. While the A edition concentrates on the area of traditional catalysis research with a focus on catalytic cycles in the field of homogeneous, heterogeneous and enzymatic catalysis as well as the aspects of catalyst production, activation and deactivation, aging and regeneration, the B edition deals with catalytic processes in the environmental sector.
Catalysis Today mainly publishes original papers and reviews on individual topics.
The Journal of Catalysis publishes, for example, studies of catalytic elementary processes on surfaces or metal complexes, from analytical methods to theoretical aspects of catalysis in all areas of catalysis.
In Kinetics and Catalysis , a Russian Journal, the focus of the publication is in the field of kinetic studies and quantum mechanical calculations.
literature
Catalysis in general
- B. Cornils, WA Herrmann , M. Muhler, C. Wong: Catalysis from A to Z: A Concise Encyclopedia. Verlag Wiley-VCH, 2007, ISBN 978-3-527-31438-6 .
- J. Hagen: Technical Catalysis. An introduction. Wiley-VCH, 1996, ISBN 3-527-28723-X , ISBN 978-3-527-28723-9 .
- M. Baerns : Basic Principles in Applied Catalysis. Springer, Berlin 2004, ISBN 3-540-40261-6 .
Homogeneous catalysis
- Arno Behr : Applied Homogeneous Catalysis. Wiley-VCH Verlag, 2008, ISBN 978-3-527-31666-3 .
- Dirk Steinborn : Fundamentals of organometallic complex catalysis. Vieweg + Teubner, 2007, ISBN 978-3-8351-0088-6 .
Heterogeneous catalysis
- P. Kripylo, K.-P. Wendlandt, F. Vogt: Heterogeneous Catalysis in Chemical Technology. German publishing house for basic industry, Leipzig 1993, ISBN 3-342-00666-8 .
- Gábor A. Somorjai : Introduction to Surface Chemistry and Catalysis . Wiley, New York 1994, ISBN 0-471-03192-5 (English).
- Gerhard Ertl , Helmut Knözinger , Ferdi Schüth , Jens Weitkamp : Handbook of Heterogeneous Catalysis. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-31241-2 .
- Gerhard Ertl: Reactions on surfaces: from atomic to complex (Nobel Lecture). (English) (PDF file; 666 kB)
Biocatalysis
- GE Jeromin, M. Bertau: Bioorganikum: Practical course in biocatalysis. Wiley-VCH, 2005, ISBN 3-527-31245-5 .
Web links
Individual evidence
- ^ Wilhelm Gemoll : Greek-German school and hand dictionary. Munich / Vienna 1965.
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis. Springer-Verlag, 2007, ISBN 978-3-8351-0088-6 ( limited preview in the Google book search).
- ^ Döbereinersche lighters. (No longer available online.) Www.gnegel.de, archived from the original on August 4, 2008 ; Retrieved January 5, 2010 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Letters between Goethe and Johann Wolfgang Döbereiner, 1810–1830, published by Julius Schiff, Verlag Böhlau, 1914.
- ^ Rutger A. Santen, P. Van Leuwen, J. Moulijn: Catalysis. Elsevier, 2000, ISBN 978-0-444-50593-4 ( limited preview in Google book search).
- ↑ W. Ostwald: Report on the work of F. Strohmann: About the heat content of the components of food. In: Z. phys. Chem. 15 (1894), pp. 705 f.
- ↑ History and development of fat hardening. (No longer available online.) Www.dgfett.de, archived from the original on August 28, 2007 ; Retrieved January 5, 2010 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Hubert Rehm: Biochemistry light. German, 2008, ISBN 978-3-8171-1819-9 , p. 23 ( limited preview in Google book search).
- ↑ Jan Koolman: Pocket Atlas of Biochemistry. Georg Thieme Verlag, 2003, ISBN 978-3-13-759403-1 , p. 90 ( limited preview in Google book search).
- ↑ James E. Huheey: Inorganic Chemistry. Walter de Gruyter, 2003, ISBN 978-3-11-017903-3 , p. 837 ( limited preview in the Google book search).
- ↑ Werner Abelshauser: The BASF. CHBeck, 2002, ISBN 978-3-406-49526-7 , p. 672 ( limited preview in Google book search).
- ↑ Reppe-Chemie at BASF ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ^ Boy Cornils, Wolfgang A. Herrmann, Manfred Rasch : Otto Roelen as a pioneer of industrial homogeneous catalysis . In: Angewandte Chemie. 106, 1994, pp. 2219-2238.
- ↑ Boy Cornils: Aqueous-Phase Organometallic Catalysis. John Wiley & Sons, 2004, ISBN 978-3-527-30712-8 , p. 639 ( limited preview in Google book search).
- ^ Thomas James Lindberg: Strategies and Tactics in Organic Synthesis. Academic Press, 2004, ISBN 978-0-12-450283-3 , p. 316 ( limited preview in Google Book Search).
- ↑ Information from the Nobel Foundation on the 2007 award ceremony to Gerhard Ertl (English)
- ^ CCR Platforming. (PDF) (No longer available online.) Www.uop.com, archived from the original on November 9, 2006 ; accessed on December 31, 2009 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ renewables Made in Germany: Biofuels in general. (No longer available online.) Www.renewables-made-in-germany.com, archived from the original on February 24, 2015 ; Retrieved January 5, 2010 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Renewable raw materials in industry. (PDF; 4.1 MB) www.fnr-server.de, accessed on January 5, 2010 .
- ↑ Max Planck Society - A powder against waste of energy. www.mpg.de, accessed on January 5, 2010 .
- ^ CM Starks: Phase-Transfer Catalysis. Springer Science & Business Media, 1994, ISBN 978-0-412-04071-9 ( limited preview in Google book search).
- ^ Ryoji Noyori: Asymmetric Catalysis: Science and Opportunities, Nobel Lecture, December 8, 2001. (PDF; 879 kB) nobelprize.org, accessed on November 21, 2009 .
- ^ Script of the University of Hanover on organocatalysis ( Memento from June 11, 2007 in the Internet Archive )
- ↑ U. Eder, G. Sauer, R. Wiechert: New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. In: Angew. Chem., Int. Ed. 1971, 10, p. 496
- ↑ Dirk Steinborn : Fundamentals of organometallic complex catalysis. Verlag BG Teubner, 2007, ISBN 978-3-8351-0088-6 , p. 8.
- ↑ PTCIssue17.pdf (application / pdf object). (PDF; 351 kB) www.phasetransfer.com, accessed on January 4, 2010 .
- ↑ Manfred T. Reetz : Biocatalysis in Organic Chemistry and Biotechnology: Past, Present, and Future. In: J. Am. Chem. Soc. 135 (2013) pp. 12480-12496.
- ^ Entry on Langmuir – Hinshelwood mechanism . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.L03451 .
- ↑ Kurt W. Kolasinski: Surface Science. John Wiley & Sons, 2008, ISBN 978-0-470-99781-9 , p. 183 ( limited preview in Google book search).
- ↑ Entry on Specific Catalysis . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S05796 Version: 2.3.1.
- ↑ Entry on General Acid Catalysis . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.G02609 Version: 2.3.1.
- ↑ W. Keim, FH Kowaldt, R. Goddard, C. Krüger: Novel coordination method of (benzoylmethylene) triphenylphosphorane in a nickel oligomerization catalyst. In: Applied Chemistry. 1978, 90, p. 493.
- ↑ , GECATS - German Catalysis Society - German Catalysis Society. www.gecats.de, accessed on December 26, 2009 .
- ↑ EFCATS - European Federation of Catalysis Societies. www.efcats.org, accessed December 26, 2009 .
- ^ IACS - the International Association of the Catalysis Societies. (No longer available online.) Www.iacs-icc.org, archived from the original on January 21, 2009 ; Retrieved January 5, 2010 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ The North American Catalysis Society. www.nacatsoc.org, accessed January 5, 2010 .























![{\ mathrm {- {\ frac {d [R1]} {dt}} = k [SH ^ {+}] [R1] [R2]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c9996175982cf896daa1b65e6634d4f550c3d000)
![{\ mathrm {- {\ frac {d [R1]} {dt}} = k_ {1} [SH ^ {+}] [R1] [R2] + k_ {2} [AH ^ {1}] [R1 ] [R2] + k_ {3} [AH ^ {2}] [R1] [R2] + \ dots}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/341fec73fb75a3804330d3792306c75dc430c664)