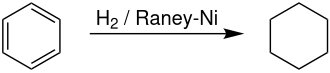Raney nickel

Raney nickel is a solid catalyst that consists of fine grains of a nickel - aluminum alloy and is used in many industrial processes. Raney nickel was developed in 1926 by the American engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils . In modern processes, Raney nickel is used as a heterogeneous catalyst in many syntheses of complex organic molecules such as B. natural substances used in hydrogenation reactions.
Raney nickel is produced by leaching an aluminum-nickel block with concentrated sodium hydroxide solution . This “activation” dissolves a large part of the aluminum from the alloy and leaves a porous structure which, due to its large surface, can strongly influence chemical reactions. A typical catalyst consists of about 85% of the mass of nickel, which corresponds to about two nickel atoms per aluminum atom.
Raney nickel is pyrophoric . i.e., it starts to burn on contact with oxygen .
Manufacturing
The alloy
Commercially, the alloy is produced by melting the active metal together - in this case nickel, iron and copper can also be used to produce other Raney-type catalysts - and aluminum in a crucible, followed by quenching . The resulting metal is ground to a fine powder and then sieved out the desired particle sizes.
The initial composition of the alloy is crucial, as the quenching causes different Ni / Al phases that behave differently in alkalis. As a result, the resulting porosity of the end product can vary widely. In most cases, the starting alloy has equal proportions by weight of nickel and aluminum, which is the same ratio that Murray Raney used when he discovered Raney nickel.
Small amounts of a third metal, such as zinc or chromium , can be used during quenching . This additional metal increases the catalytic activity and is therefore referred to as a “ promoter ”. However, the promoter changes the alloy and its phase diagram to a triple alloy , which, in addition to a different method of deterrence, also results in changed behavior during activation.
The activation process
The porous structure of the catalyst is created by dissolving the aluminum with caustic soda. The simplified lye reaction is given by the following reaction equation:
The sodium aluminate (Na [Al (OH) 4 ]) that occurs here forces the use of concentrated sodium hydroxide solutions to prevent the precipitation of aluminum hydroxide in the form of bayerite. The alkalis have concentrations of up to 5 mol · l −1 . The bayerite can clog the pores of the structure formed and, due to the resulting reduced surface area, negatively affect the efficiency and activity of the catalyst.
The temperature of the liquor also has a significant influence on the surface properties of the catalyst, conventional temperatures of 70 to 100 ° C range. In the case of Raney nickel and Raney-type catalysts, increasing temperature during the activation process reduces the resulting surface area. This is due to structural deformations within the alloy that take place in a similar way to sintering .
Before storage, the catalyst is washed with distilled water at room temperature in order to remove remaining traces of sodium aluminate . Low-oxygen water is preferred for storage in order to prevent oxidation of the catalyst, which accelerates its aging and reduces the catalytic effect.
properties
Macroscopically, Raney nickel looks like a finely divided gray powder. Microscopically, however, each particle resembles a three-dimensional grid, the openings of which - pores of irregular size and shape - are mainly created by the leaching. Raney nickel is thermally and structurally stable and has a large BET surface. These properties mainly result from the activation process and contribute to the comparatively high catalytic effectiveness.
During activation, the aluminum is released from the NiAl 3 and Ni 2 Al 3 phases of the alloy; the remaining aluminum is mostly in the form of NiAl. This removal of the aluminum from specific phases is known as “selective leaching”. The NiAl phase supports the structural and thermal stability of the catalyst, which is therefore resistant to decomposition. This resistance allows Raney nickel to be stored and used for long periods of time; however, mostly fresh preparations are used for laboratory use. For this reason, commercial Raney nickel is offered in both “active” and “inactive” form.
The surface is often determined by means of BET measurements, for which a gas, such as hydrogen , is used , which is mainly adsorbed by metallic surfaces . Such measurements demonstrated that almost the entire exposed surface of the particles consists of nickel. Since nickel is the active metal of the catalyst, there is a large area available for chemical reactions, which is reflected in the catalytic effectiveness. Commercially available Raney nickel has an average nickel surface area of 100 m² per gram.
Due to the high effectiveness and the adsorption of hydrogen within the pores, Raney nickel is a suitable catalyst in many hydrogenation reactions. Its stability - for example the fact that it does not decompose at high temperatures - also allows a wide range of reaction conditions. With the exception of mineral acids - for example hydrochloric acid - Raney nickel is hardly soluble in most laboratory solvents and its high density, which is between 6 and 7 g · cm −3 , facilitates the separation of liquid phases after a reaction.
The particle size is normally 20 to 50 µm, in special cases also 10 µm or up to 90 µm.
Applications
Raney nickel is used in a wide variety of industrial processes and laboratory syntheses because of its stability and effectiveness at room temperature. It is typically used in the reduction of components with multiple bonds - such as alkynes , alkenes , nitriles , polyenes , aromatics and substances of the carbonyl group . In addition, Raney nickel reduces heteroatom-heteroatom bonds such as organic nitro compounds and nitrosamines . The alkylation of amines and the amination of alcohols represent another area of application.
An example of the use of Raney nickel in industry is the following reaction, in which benzene is reduced to cyclohexane . Reduction of the aromatic structure of the benzene ring is difficult to achieve with other chemical methods, but it can be achieved with Raney nickel. Other heterogeneous catalysts, such as those that use elements of the iron-platinum group , can also be used, but are usually more expensive than Raney nickel. After this reaction, cyclohexane can be used to synthesize adipic acid , a raw material used in the production of polyamides such as nylon.
When reducing carbon-carbon double bonds, Raney nickel adds hydrogen ( addition ). In addition to being used as a catalyst, Raney nickel can also be used as a reagent for desulphurising organic compounds. For example, thioacetals are reduced to hydrocarbons :
Nickel sulphide precipitates, while ethane can escape as a gas. Similar transformations are the Clemmensen reduction and the Wolff-Kishner reaction . Phosphine sulfides can also be desulfurized to the free phosphines with Raney nickel at room temperature.
Individual evidence
- ^ Raney, Murray (1927). " Method of producing Finely Divided Nickel ( Memento of the original dated February 25, 2006 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this note. (PDF file ; 223 kB) ". U.S. Patent 1628190 , issued May 10, 1927.
- ↑ a b c d e f g Ertl, Gerhard; Knözinger, Helmut (Eds.) (1997). Preparation of Solid Catalysts , Weinheim: Wiley. ISBN 3-527-29826-6
- ↑ a b A.J. Smith and DL Trimm (2005). Annual Reviews in Materials Research , 35 , 127-142.
- ↑ Grace: Raney® Catalyst Slurry Grades ( Memento from August 28, 2008 in the Internet Archive )
- ^ Organic Syntheses (2005). "Raney nickel usage in Organic Syntheses " ( Memento June 5, 2009 in the Internet Archive ). Last accessed January 25, 2006.
- ↑ Page, GA; Tarbell, DS: β- (o-Carboxyphenyl) propionic acid In: Organic Syntheses . 34, 1954, p. 8, doi : 10.15227 / orgsyn.034.0008 ; Coll. Vol. 4, 1963, p. 136 ( PDF ).
- ↑ Robinson, Jr., HC; Snyder, HR: β-Phenylethylamine In: Organic Syntheses . 23, 1943, p. 71, doi : 10.15227 / orgsyn.023.0071 ; Coll. Vol. 3, 1955, p. 720 ( PDF ).
- ↑ Schwenk, E .; Papa, D .; Hankin, H .; Ginsberg, H .: γ-n-Propylbutyrolactone and β- (Tetrahydrofuryl) propionic acid In: Organic Syntheses . 27, 1947, p. 68, doi : 10.15227 / orgsyn.027.0068 ; Coll. Vol. 3, 1955, p. 742 ( PDF ).
- ↑ Enders, D .; Pieter, R .; Renger, B .; Seebach, D .: Nucleophilic α-sec-aminoalkylation: 2- (diphenylhydroxymethyl) pyrrolidene In: Organic Syntheses . 58, 1978, p. 113, doi : 10.15227 / orgsyn.058.0113 ; Coll. Vol. 6, 1988, p. 542 ( PDF ).
- ^ Rice, RG; Kohn, EJ: N, N'-Diethylbenzidene In: Organic Syntheses . 36, 1956, p. 21, doi : 10.15227 / orgsyn.036.0021 ; Coll. Vol. 4, 1963, p. 283 ( PDF ).
- ↑ a b c Solomons, TW Graham; Fryhle, Craig B. (2004). Organic Chemistry (8th Edn.) , Wiley International Edition. ISBN 0-471-41799-8
- ↑ Gassman, PG; van Bergen, TJ: Indoles from anilines: Ethyl 2-methylindole-5-carboxylate In: Organic Syntheses . 56, 1977, p. 72, doi : 10.15227 / orgsyn.056.0072 ; Coll. Vol. 6, 1988, p. 601 ( PDF ).
- ↑ Wenjun Tang, Weimin Wang, Xumu Zhang: Phospholane – Oxazoline Ligands for Ir-Catalyzed Asymmetric Hydrogenation . In: Angewandte Chemie International Edition . tape 42 , no. 8 , February 24, 2003, p. 943-946 , doi : 10.1002 / anie.200390251 .
![{\ displaystyle {\ ce {2 Al + 2 NaOH +6 H2O -> 2 Na [Al (OH) 4] + 3 H2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0b41ea75b9d1c286913dd4910879cbce9a28e868)