Alkynes
Alkynes (formerly acetylenes and acetylenic hydrocarbons ) are chemical compounds from the group of aliphatic hydrocarbons that have at least one carbon-carbon triple bond (R – C≡C – R) in the molecule. The alkynes with only one such triple bond form a homologous series with the general empirical formula C n H 2 n −2 (with n = 2, 3, 4, ...), which begins with ethyne .
Compounds with two or more carbon-carbon triple bonds are called polyynes ; in a broader sense, acyclic hydrocarbons with several carbon-carbon triple bonds can be counted among the alkynes.
Cyclic compounds with a carbon-carbon triple bond, on the other hand, are counted among the cycloalkynes and aromatic hydrocarbons with a formal triple bond in the ring are called arynes .
history
For the first time acetylene (ethyne), the simplest alkyne, was obtained in 1836 from Edmund Davy , a chemistry professor at the Royal Dublin Society and cousin of the famous English chemist Humphry Davy . When trying to represent potassium in metallic form, he obtained ethyne by heating potassium salts such as potassium acetate or potassium carbonate with charcoal , followed by the reaction of the potassium carbide formed with water. However, the discovery was forgotten. In 1862 the German chemist and doctor Friedrich Wöhler succeeded in preparing acetylene by reacting water with calcium carbide . In 1863, the French chemist Marcelin Berthelot succeeded in depicting the elements using the arc between graphite electrodes in a hydrogen atmosphere. He named the gas acetylene. In 1895, Henry Le Chatelier discovered that acetylene burns with oxygen with a very hot flame. This laid the foundation for acetylene welding and cutting.
Homologous series
Here are the most important alkynes from ethyne (C 2 H 2 ) (acetylene) to decyne (C 10 H 18 ) with their names and molecular formula:
- Ethyne : C 2 H 2
- Propyne : C 3 H 4
- 1-butyne : C 4 H 6
- 2-butyne : C 4 H 6
- Pentyne : C 5 H 8
- Hexyne : C 6 H 10
- Heptyne : C 7 H 12
- Octyne : C 8 H 14
- Nonin : C 9 H 16
- Decin : C 10 H 18
nomenclature
The naming of the alkynes according to the IUPAC rules is based on the names for the alkanes . The stem name for the alkyne is chosen from the root word of the alkane with the same number of carbon atoms and the suffix -an is replaced by -in . In the case of branched alkynes, the longest possible carbon chain with the triple bond gives the stem name. Alkynes have a higher priority than alkenes. The first two representatives of the homologous series of this group of substances are ethyne (H – C≡C – H) and propyne (H – C≡C – CH 3 ). The position of the triple bond is described with a number so that it is as small as possible, e.g. B. 1-butyne (H-C≡C-CH 2 -CH 3 ) and 2-butyne (H 3 C-C≡C-CH 3 ). If the carbon chain contains several triple bonds, one adds di , tri , tetra etc. in front of the syllable in the name . An alkyne with five (Greek penta ) carbon atoms and two triple bonds after the 1st and 4th carbon atom is given the IUPAC name 1,4-pentadiyne (H – C≡C – CH 2 –C≡C – H ). The structural isomeric 1,3-pentadiyne has the following structure: H – C≡CC≡C – CH 3 .
Electronic structure
The triple bond of the alkynes consists of a sp hybrid bond and two orthogonal p bonds. These two orthogonal p-bonds (orthonormal basis) form two rotation-invariant orbitals, which z. B. can be demonstrated by IR spectroscopic investigations on W 2 (CO) 6 . Due to the higher proportion of s orbitals in the sp hybrid orbitals, the probability of the electrons of the CH bond near the carbon nucleus is greater than with alkenes (sp 2 ) and alkanes (sp 3 ), whereupon the CH acidity is more terminal Alkynes based. Therefore, the pKa value of ethine of 25 is significantly lower than that of ethene (44) or ethane (50). With strong bases, a terminal carbon atom can be deprotonated in lower alkynes and z. B. be replaced by a metal atom. Salts of the alkynes are called acetylides . Apart from the weak CH polarity due to the shortened CH bond, alkynes are relatively non-polar. From the electronic structure of the C≡C triple bond it follows that the carbon atoms of the triple bond and the two atoms directly linked to these carbon atoms are aligned in a line (linear). The carbon-carbon distance of a triple bond is 120 pm and is thus shorter than the distance of a C = C double bond.
Spectroscopic properties
1 H-NMR
In 1 H-NMR , alkynyl hydrogen atoms are not as strongly deshielded as alkenyl hydrogen atoms, since in the rotationally symmetrical triple bond the external magnetic field induces a ring current whose magnetic field is opposite to the external one. Since the triple bond transmits the spin-spin coupling well, the 4 J-couplings over four bonds and the 5 J-couplings over five bonds usually have coupling constants of 1 to 3 Hz. For long-distance couplings, this is a relatively high value, therefore it is often characteristic of the alkynyl group .
Infrared spectroscopy
In addition to 13 C-NMR spectroscopy, infrared spectroscopy is a useful method for identifying a terminal alkyne, as a strong characteristic band for the CC stretching vibration is shown at around 2100 cm −1 .
Extraction and preparation of ethine
- Extraction and subsequent hydrolysis of calcium carbide
- Calcium oxide and coke react to form calcium carbide and carbon monoxide .
- Calcium carbide and water react to form ethyne and calcium hydroxide .
- Dimerization and dehydrogenation of methane
- Methane reacts to ethyne and hydrogen under certain conditions.
- Partial methane oxidation
- Methane oxidizes to ethyne, carbon monoxide and hydrogen.
Reactions
Hydrogenation
Hydrogenation with normal catalyst
Alkynes can be hydrogenated to alkanes with hydrogen with the aid of conventional catalysts such as platinum or palladium.
Hydrogenation with a poisoned catalyst
With a poisoned catalyst, the Lindlar catalyst , the alkyne only reacts to form the alkene. Only ( Z ) -alkenes ( cis ) are formed, since the hydrogen atoms approach the alkyne from the same side and react.
Formation of alkynylides
Since terminal alkynes, as already mentioned, are CH acids, they can react to form the so-called alkynylides. With alkali metals in liquid ammonia they form the alkali alkynylides. The insoluble heavy metal alkynylides are formed in aqueous solutions of silver (I) and copper (I) salts. However, when dry, these are highly explosive!
Electrophilic addition
The addition to a triple bond is more exothermic than the addition to a double bond.
Nevertheless, alkynes are less reactive than alkenes towards electrophilic reagents. The apparent contradiction can be explained by the stability of the intermediate carbenium cations:
The alkyl cation is much more stable than a vinyl cation, the addition reaction of electrophiles to alkynes is kinetically inhibited (= higher activation energy).
Halogenation
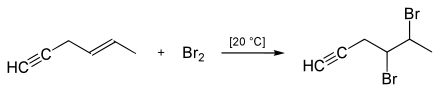
According to Hallab's method, halogenation is applied as follows: The CC triple bond is less nucleophilic than the carbon-carbon double bond, which is why the halogens do not add spontaneously to the triple bond. This only happens when the halogen-halogen bond is polarized with the aid of a Lewis acid, for example FeCl 3 or AlCl 3 . This produces the ( E ) -1,2-dihaloalkene , stereoselectively .
Therefore, in the presence of a carbon-carbon triple bond, the carbon-carbon double bond can be selectively halogenated as long as no Lewis acid is added.
Hydrohalogenation
Hydrogen halide can be added to alkynes in the first stage to form vinylogous haloalkene. However, these only react further to form the dihalogens under more drastic conditions, since these are not very reactive. According to Markovnikov's rule, 1,1-dihaloalkanes are formed here.
The hydrochlorination of ethyne to vinyl chloride was of great technical importance, since vinyl chloride is the monomeric building block for the production of PVC .
Hydration
Water is added to ethyne to ethenol in the presence of catalysts in an acidic environment. This tautomeric form finally rearranges to acetaldehyde ( keto-enol tautomerism ).
Terminal alkynes, on the other hand, rearrange after hydration (also here according to the Markovnikoff rule ) to give the methyl ketones.
Natural occurrence
Alkynes are not very common in nature (only 1000 compounds are known) and only a few of them are physiologically active in their own organism. The rest mostly act as fungicides or as defensive poisons or mucous membrane irritants. The skin of poison dart frogs, for example, secretes histrionicotoxin , a substance that contains two alkyne groups and protects the frog from mammals and reptiles. Enediyne antibiotics form a large group of biologically active alkynes. Natural products , the structural motif the enediyne unit have often act cytotoxic to human tumor cell lines and thus represent potential chemotherapeutic agents. One example is that of Streptomyces carcinostaticus secreted neocarzinostatin .
meaning
Only ethyne (common name acetylene) and propyne are of technical importance ; they are used as welding gas, among other things, because the flames of these become extremely hot (up to 3100 ° C). Ethyne is of great importance in the chemical industry for the production of many other compounds such as acrylic acid or acrylamide . Also, 2-butyne-1,4-diol is a precursor for the production of tetrahydrofuran (THF).
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry , 1st Edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 259-269.
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 91-100.
- Morrison / Boyd : Textbook of Organic Chemistry , 3rd Edition, Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6 , pp. 629-648.
- Streitwieser / Heathcock : Organic Chemistry , 1st Edition, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 363-383.
- K. Peter C. Vollhardt , Neil E. Schore : Organic Chemistry , 4th Edition, Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31380-8 , pp. 631-661.
Web links
Individual evidence
- ↑ Entry on alkynes . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00236 Version: 2.3 ..
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexicon of Chemistry , Spectrum Academic Publishing House, Heidelberg 2001.