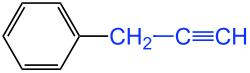Alkynyl group
In organic chemistry, an alkynyl radical or alkynyl group is a substituent that contains a carbon-carbon triple bond ( acetylenic structural element). Formally, an alkynyl radical is derived from an alkyne hydrocarbon by removing an H atom . In analogy to alkyl residues , the carbon atom to be linked to another molecule (residue) (with the so-called free valence , “yl”) is given the number “1”. From here you count and number to the end of the longest carbon chain that contains the triple bond.
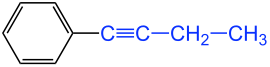
The simplest alkynyl radical is logically derived from the C 2 hydrocarbon ethyne ( acetylene ); it is therefore called " ethinyl ". From the C 3 hydrocarbon propyne, there are isomeric alkynyl radicals, depending on the position of the triple bond. Therefore this must be indicated after the number. A distinction is therefore made between the 1-propynyl and 3-propynyl groups. The 3-propynyl radical is also referred to by the old common name “ propargyl ”, but this is not IUPAC- compliant. In the more recent literature a more detailed variant is often chosen by using several digits: e.g. B. 1-propyn-1-yl and 2-propyn-1-yl (= propargyl). However, these names are more awkward to pronounce. Three isomeric C 4 alkynyl residues (butynyl residues) are shown in the figure.
literature
- H. Grünewald (Ed.): International rules for chemical nomenclature and terminology . German edition, Vol. 1, Verlag Chemie, Weinheim 1975.
Individual evidence
- ^ Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 91.