Ethine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 | |||||||||||||||
| Brief description |
colorless, flammable gas, in pure form with a slightly ethereal smell, but for technical reasons mostly with a garlic-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 26.04 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Sublimation point |
−84.03 ° C |
|||||||||||||||
| Vapor pressure |
4,336 M Pa (20 ° C) |
|||||||||||||||
| solubility | ||||||||||||||||
| Dipole moment |
0 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 1000 ml m −3 or 1080 mg m −3 |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
226.73 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ethine (rare: ethine ; common name : acetylene from Latin acetum 'vinegar' and ancient Greek ὕλη hyle 'wood, fabric') is a colorless gas with the empirical formula C 2 H 2 . It is the simplest member of the homologous series of alkynes .
Ethine is of great industrial importance. It is used as a starting compound in the large-scale production of important basic chemicals. For oxy- fuel welding , oxy-fuel cutting and brazing , it is usually transported in gas cylinders and bound in a solvent (e.g. acetone or dimethylformamide ) contained therein . In this dissolved form, it is also called dissous gas ([ dɪˈsuˌɡaːs ], from French dissoudre , dissous : to dissolve).
Ethine is the gas with the highest possible carbon content. The result is a particularly high calorific value , which means that the highest combustion temperatures can be achieved with ethine. If ethine flowing out of nozzles burns in ambient air, glowing carbon particles - which only exist temporarily - result in a particularly bright flame that emits a simple, good light source. If the flame is quenched on a cooling surface, fine, pure soot can be obtained.
history
Ethin was discovered by Edmund Davy in 1836 when he heated calcined potassium tartrate with charcoal to make potassium ; however, he only wrote his observations in his laboratory journal . In 1862, ethin was first produced from calcium carbide by Friedrich Wöhler and documented publicly. In the same year Marcellin Berthelot was able to produce ethyne from the elements carbon and hydrogen. As early as 1866, Berthelot observed that ethyne cyclizes to benzene at high temperatures on metal surfaces . In 1881 it was observed by Michail Kutscheroff that ethanal is accessible from ethyne by water attachment. Carbide lamps using ethine as a fuel gas appeared in the 1890s.
Around 1930, Reppe chemistry (ethyne chemistry) developed in Germany . Since Walter Reppe was able to minimize the risk of explosion from the pressurized ethyne, many new reactions could be carried out industrially. Reppe's windowless laboratories are located on the top floors of the building on the BASF AG site in Ludwigshafen am Rhein .
Reppe chemistry is summarized in four main reactions: vinylation , cyclization , ethynylation and carbonylation of ethyne, all of which occur at higher pressures. However, ethyne was largely displaced by ethene in organic synthesis after the Second World War , because ethyne is more expensive to produce, while ethene is obtained in large quantities in industrial processes since petrochemicals began to rely on petroleum after the Second World War . Up until 1950, ethyne from coal was an important organic starting substance alongside the aromatics in coal tar. In the 1980s, a pilot reactor for the extraction of ethyne from coal was built in the old federal states (see arc plasma reactor). It is still used today for a significant number of syntheses.
Giulio Natta polymerized ethyne for the first time in 1958 to produce polyethine , the first semiconductor polymer , which, however, is unstable in air. Alan Heeger and Alan MacDiarmid from the USA as well as the Japanese Hideki Shirakawa showed in 1976 that doping the polyethine with oxidizing agents leads to a very strong increase in electrical conductivity. The three scientists received the Nobel Prize in Chemistry in 2000 for their work in the development of electrically conductive polymers.
Acetylene centers
The first acetylene center was built in 1897 in Totis in Hungary by the Vienna Acetylene Company. In southern Germany, the Keller & Knappich company (now KUKA ) built apparatus and control centers in Augsburg. In total there were 29 acetylene centers around 1901 and by 1904 there were already around 60–70 towns and cities with acetylene lighting in Germany. In addition to residential buildings that were connected to the pipe network, street lights were also supplied.
Occurrence and manufacture
There is no natural occurrence of ethine on earth. Outside Earth, it has been detected in Jupiter's atmosphere as well as in interstellar matter .
The annual world production in 1998 was 122,000 tons. On an industrial scale, ethyne is produced by means of high-temperature pyrolysis (see also HTP process ) of light or medium petroleum fractions or natural gas at 2,000 ° C. After the pyrolysis , the resulting gas mixture is quickly cooled to below 200 ° C ( quenched ) in order to avoid further decomposition into elemental carbon and hydrogen. An ethyne- ethene mixture is obtained from which the ethyne is fractionated. The heat transfer can take place in different ways; the most modern method, the hydrogen - arc pyrolysis, an older, more frequently used method is the arc pyrolysis.
Another method of making ethyne is by reacting calcium carbide with water:
The lime residue (Ca (OH) 2 ) from this reaction is called blue lime .
The resulting ethyne has a garlic-like odor due to impurities (e.g. monophosphine ).
The direct production from hydrogen and carbon is technically insignificant. It takes place in an electric arc at around 2,500 ° C.
In addition, ethene, which is obtained from petroleum processing, is dehydrated to ethine . It is also produced by the incomplete combustion of methane , but this process is not economical.
Highly pure ethyne can be produced from commercially available technical ethyne (purity approx. 99.5%) by passing the technical gas through an adsorber column filled with fine-grained activated carbon and a molecular sieve . The column material removes trace contaminants, e.g. B. monophosphane (PH 3 ), monoarsane (AsH 3 ), ammonia (NH 3 ) and hydrogen sulfide (H 2 S), from the Rohalkin and also removes residues of the solvent in which the gas is dissolved in the pressurized gas cylinder . Fine-pored filters prevent fine dust from being carried away. The purity of the ethyne leaving the purification column is between 99.99% and 99.9999%. The process developed in Japan is described in detail in patent application US4863493 from 1989. The patent application JP2004148257 from 2004 presents a portable system for the production of ultrapure ethyne from technical ethyne, which uses the same cleaning technique.
Properties and dangers

Physical Properties
Ethyne is a colorless gas under standard conditions. Since the triple point is above normal pressure, like carbon dioxide , ethyne can not be liquefied at normal pressure. Solid ethyne occurs in two polymorphic crystal forms. A cubic crystal lattice is formed below −140.15 ° C. The orthorhombic crystal form existing above the transition point sublimes under normal pressure at −83.8 ° C. The enthalpy of conversion between the two polymorphic forms is 2.54 kJ · mol −1 .
The solubility of ethyne in water is only 1.23 g / kg at atmospheric pressure, whereas the solubility in ethanol and acetone (27.9 g / kg under standard conditions) is very good. The latter is used to transport ethine in pressurized gas cylinders. Most pressurized gas cylinders for ethyne are nowadays filled with a porous mass of calcium silicate hydrate, into which acetone is added, which in turn can dissolve ethyne in large quantities and thus store it. In the event of a flashback (welding) through the valve, the porous mass prevents a possible explosion-like decomposition of the ethine in the bottle.
The calorific value is 57,120 kJ / m³ ( normal ) or 48.5 MJ / kg. Ethine burns in the air with a bright, sooty flame. When burned with air, the flame becomes approx. 1900 to 2300 ° C, when burned with pure oxygen up to 3200 ° C (used in autogenous welding ). This is one of the highest (flame) temperatures that can be achieved by chemical reaction (from room temperature) .
Acetylene forms a solid, stoichiometric hydrate (a clathrate analogous to methane hydrate ) with the composition C 2 H 2 · 5.75H 2 O in the temperature range between −4 ° C and 16 ° C above a pressure of 5 bar .
structure
The molecule is due to the sp- hybridisation entirely of carbon atoms linearly built; all bond angles are therefore 180 °. The carbon triple bond is 120 picometers long , the carbon-hydrogen bond is 106 picometers long.
The triple bond in ethyne consists of a sp hybrid bond (σ bond) and two orthogonal π bonds. The latter form two rotationally invariant orbitals. Due to the strong s-character of the sp hybrid orbitals, the probability of the electrons of the C – H bond being in the vicinity of the carbon is greater than with ethene with sp 2 -hybridized carbon or with ethane with sp 3 -hybridized carbon. Therefore, the C-H bond is relatively acidic (pK s = 25). A number of ethyne salts, so-called acetylides , are known.
Thermodynamic properties
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.66141, B = 909.079 and C = 7.947 in the temperature range of 214.6 K to 308.3 K. The temperature dependence of the molar heat capacity of gaseous acetylene can be calculated using the Shomate equation c p = A + B t + C t 2 + D t 3 + E / t 2 (c p in J mol −1 K −1 , t = T / 1000, T in K) with A = 40.68697, B = 40.73279, C = −16.17840, D = 3.669741 and E = - 0.658411 in the temperature range from 298 to 1000 K.
The most important thermodynamic properties are listed in the following table:
| size | Formula symbol | value | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 gas | 226.73 kJ mol −1 | |
| Enthalpy of combustion | Δ c H 0 gas | −1255.6 kJ mol −1 | 298.15K |
| Heat capacity | c p | 44.04 J mol −1 K −1 (298.15 K) | than gas |
| Triple point | T triple | 192.4 K | = -80.75 ° C. Lt. Gesture: -80.6 ° C. |
| Triple point | p triple | 1.2825 bar | |
| Critical temperature | T c | 308.3 K | |
| Critical pressure | p c | 61.38 bar | |
| Critical volume | V c | 0.1122 l mol −1 | |
| Critical density | ρ c | 8.91 mol·l −1 |
Safety-related parameters
Ethine, like all lower hydrocarbons, forms easily flammable mixtures with air. In addition, as a result of its endothermic character, the compound tends to explosively self-decay into the elements. In addition to the properties based on combustion processes, the safety-related characteristic data can be influenced by the decomposition reaction as the concentration of ethyne increases. The lower explosion limit (LEL) of 2.3% by volume (24 g / m 3 ) is safely based on pure combustion. Information on the upper explosion limit (UEL) of 82% by volume, resulting from correctly applied, standardized test methods, can be justified by self-decomposition because of the low oxygen concentration required for combustion. Since no oxygen is required for self-decomposition, the upper explosion limit is set to 100% by volume, since the physico-chemical cause is of secondary importance in the result or extent of an explosion.
The maximum explosion pressure in a combustion reaction is 11.1 bar. The minimum ignition energy is extremely low at 0.019 mJ and is comparable to that of hydrogen . The limit gap width was determined to be 0.37 mm. This results in an assignment to explosion group IIC. The ignition temperature is 305 ° C. This value applies to a mixture of 38% by volume ethine in air at atmospheric pressure. The substance therefore falls into temperature class T2. An air content of around 10% by volume is necessary for effective self-ignition. Self-ignition tests in quartz tubes show a significant drop in the ignition temperature from around 550–600 ° C to around 300–350 ° C in the range from 10% air by volume, which can be interpreted as a transition from self-decay to an oxidative combustion process. The temperature of the self-decomposition of acetylene is 635 ° C under these conditions at atmospheric pressure.
The self-decomposition of acetylene can be triggered by various sources such as heat of reaction, hot surfaces, electrostatic discharges, heat of compression or mechanical shock waves. If one of the ignition sources mentioned is present, acetylene can decompose detonatively under normal pressure from 160 ° C. In contrast, a mixture of 72 vol.% Nitrogen and 28 vol.% Acetylene is stable at the same temperature up to a pressure of 20 bar. The stability limit pressures as a function of the nitrogen content have been determined experimentally. With a sufficiently strong ignition source, the decomposition of pure acetylene can be triggered at a pressure of 0.8 bar. The tendency to self-disintegration depends, among other things, on the geometry of the vessels. The tendency to disintegrate in pipeline systems and pipelines has been extensively investigated. Wall surfaces that trap radicals and absorb energy when a chain reaction begins can stabilize the gas phase. Overall, the self-disintegration of acetylene depends on a large number of factors, which makes it necessary to consider the individual case when dealing with this substance. The handling of acetylene, especially under pressure, is dangerous and requires strict compliance with the technical regulations.
Due to its tendency to deflagration or detonation, acetylene cannot be stored in liquefied form in pressure vessels or pressurized gas cylinders like other gases. Here it is dissolved in solvents such as acetone or dimethylformamide , in which it is highly soluble . This solution is in turn distributed in a porous material. The total pressure in the container depends on the solvent / acetylene ratio and the temperature. So z. B. for an acetylene-acetone ratio of 1: 1 at 20 ° C a pressure of 25 bar.
Ethyne must not come into contact with materials containing copper, as otherwise the highly explosive copper acetylide can form. The same applies to silver.
If ethine is inhaled, it leads to dizziness and indifference . However, a maximum workplace concentration is not specified.
Fire
Gas cylinders (usually made of steel) with ethyne ( acetylene cylinders) dissolved under pressure , which have even been exposed to significant or uncertain heating in places, are considered to be very dangerous, as a decomposition reaction that cannot be observed from the outside can develop heat of reaction can heat the contents further, which can lead to the bottle bursting due to excess pressure. Parts of the bottle could be thrown through the air with great energy and also break through thinner walls. The risk can be estimated by observing the course of the fire and measuring the radiation temperature. The first measure - if possible at an early stage with low risk - is to remove the bottle from the area of the flame and take it outside. Second, water is used to keep the bottle cool for a long time from a safe distance. Thirdly, the bottle is ideally opened at certain points by being shot at with special ammunition so that ethine - outdoors - flows out and reduces the internal pressure. The gas - a little lighter than air - is flared so as not to ignite elsewhere.
Chemical properties and reactions
In its pure state, ethine smells slightly ethereal and is non-toxic. From technical calcium carbide (CaC 2 ) - z. B. from welding carbide - produced ethine often has an unpleasant, slightly garlic-like odor, which comes from impurities. Most of these are phosphine (PH 3 ), arsine (AsH 3 ), ammonia (NH 3 ) and hydrogen sulphide (H 2 S), which are produced from calcium carbide during industrial production. The appearance of divinyl sulfide in ethyne during its manufacture from calcium carbide can be attributed to the presence of traces of calcium sulfide in commercial calcium carbide, which reacts with ethyne to form divinyl sulfide.
Ethine is a CH-acidic compound. It is having a pK s value of 25 but considerably less acidic than water (pK s value of 15.7) and acidic than ammonia (pK s value 35). This means that it practically does not dissociate in water. Under more drastic conditions (e.g. with sodium amide, NaNH 2 in liquid ammonia) ethyne is easily deprotonated and forms acetylides :
The slight dissociation in aqueous ammoniacal solution, however, is sufficient for the acetylide anion formed to react with transition metal cations, e.g. B. Ag + or Cu 2+ , to very poorly soluble metal acetylides, such as. B. with Ag + to silver acetylide or with Cu 2+ to copper acetylide . The reaction equilibrium is shifted in the product direction by the precipitation of the acetylides:
Such metal acetylides are extremely sensitive to impact when dry and easily explode.
At high pressure, ethyne breaks down into soot and hydrogen:
It can be hydrogenated to ethene and finally to ethane :
With many catalysts, this hydrogenation cannot be stopped at the alkene stage. With some palladium or nickel catalysts, however, ethene synthesis from ethyne succeeds with careful selection of the reaction conditions.
Ethine reacts with chlorine to form carbon and hydrogen chloride :
The addition of halogens is also possible. The pi electron cloud of the ethyne polarizes the attacking halogen molecule and shares its pi electrons with it, creating a three-membered ring consisting of two carbon atoms and a (positively charged) halogenium ion (the charge is distributed over the entire ring); in addition, a halogen atom with a negative charge is released. In the second step, this acts as a nucleophile and attacks the unstable ring, creating a vicinal dibromide. The first addition with chlorine forms 1,2-dichloroethene and the next addition tetrachloroethane . The addition of halogen to ethyne takes place more slowly than with ethene, because the C≡C triple bond is less nucleophilic than a C = C double bond:
Similarly, it can react with bromine to discolour the reaction solution.
Vinyl halides can be produced with hydrogen halides. This is how ethyne reacts with hydrogen chloride to form vinyl chloride :
It can be hydrated with the help of a catalyst to vinyl alcohol , which is the enol of acetaldehyde and tautomerizes to it .
Ethyne can - with the help of catalysts - be cyclized to styrene or cyclooctatetraene ; 4 ethyne molecules are converted to the end product:
Butenine (vinylacetylene) is formed under catalysis by copper (I) chloride in the aqueous phase .
In carbonylation , ethyne is reacted with carbon monoxide and water over catalysts such as nickel tetracarbonyl to form unsaturated carboxylic acids . This is how propenoic acid is formed in the above reaction :
Alcohols and carboxylic acids can be added to ethyne. Adding alcohols results in vinyl ethers , carboxylic acids result in vinyl esters :
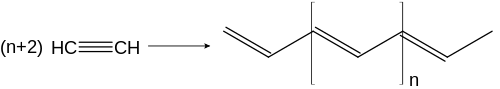
When heated over Ziegler-Natta catalysts, ethyne polymerizes to polyethine (in the picture trans-polyethine). It can polymerize to cuprene on copper catalysts .
use
During the time of the so-called "acetylene chemistry", ethine was the most important chemical raw material. After 1965, the ethyne capacity was practically no longer increased, since many acetylene derivatives were made available from the cheaper raw materials ethene ( ethylene ) and propene ( propylene ).
| product | Competing raw material | Procedure |
|---|---|---|
| Acrylonitrile | Propene | Ammoxidation |
| acetaldehyde | Ethene | Oxidation with O 2 or air |
| Vinyl chloride | Ethene | Oxychlorination |
| Vinyl acetate | Ethene | Oxidation with O 2 |
| Tetrahydrofuran | Maleic anhydride | Hydrogenation |
About 80% of the ethyne is used for organic synthesis . The addition of hydrogen halides produces vinyl halides and polyvinyl halides , for example vinyl chloride or polyvinyl chloride. By addition of acetic acid is vinyl acetate and polyvinyl acetate prepared by the addition of ethanol vinyl ethers and polyvinyl ethers . In addition, cyclooctatetraene , acrylic acid , acetic acid, 1,3- and 1,4- butanediol , propargyl alcohol , 2-butyne-1,4-diol , vinylethine , succinic acid , neoprene , chloroprene , vinyl ester , polyvinyl ester , higher alcohols , and monochloroethanoic acid are made from ethyne synthesized. The polymers produced in particular are of industrial importance. Benzene , 1,3-butadiene , ethanol, acrylonitrile and polyacrylonitrile , vinyl halides, acrylic acid and acetaldehyde are more rarely produced from ethyne .
The acetylene black obtained from ethyne is used as a rubber additive in the production of black rubber or for the production of printing ink and in batteries . Due to the high binding energy of the triple bond , ethyne was used for lighting purposes ( carbide lamp ) and is nowadays often used as dissous gas for oxy- fuel welding and oxy- fuel cutting . In the trade it is sold in maroon (formerly yellow) bottles. Until the 1950s, pure ethine mixed with 60% oxygen , called narcylene , was used as an inhalable anesthetic . However, when it exploded, it was no longer used. Ethynylation plays a role in the industrial synthesis of terpenes , which are mainly used as fragrances and aromas; even in the basic step for all terpene syntheses, ethynylation is carried out with acetone in the presence of a base to give 3-butyn-2-ol , The ethynylation is also found again and again in further higher steps. Ethynylation also takes place in the synthesis of vitamin A : in one step, β- ionone is ethynylated to ethynylionol.
Ethine is also used in microelectronics and microtechnology . Here it serves z. B. for the deposition of diamond , graphite or polyacetylene layers and for the production of nanotubes .
In analytical chemistry , ethyne is used as fuel for the gas flame of the atomic absorption spectrometer . The components of a sample are atomized by the high flame temperature.
proof
The detection of ethyne is possible by introducing the gas into an ammoniacal silver (I) or copper (I) salt solution, whereby poorly soluble acetylides precipitate:
literature
- Julius A. Nieuwland, RR Vogt: The Chemistry of Acetylene. Reinhold, New York 1945.
- SA Miller: Acetylene, its Properties, Manufacture and Uses, Vol. 1. Ernest Benn, London 1965.
- Walter Reppe: Chemistry and technology of the acetylene pressure reactions. Verlag Chemie, 1952.
- Heinz Gunter Viehe: Chemistry of Acetylenes. M. Dekker, New York 1969.
- Paul Hölemann, Rolf Hasselmann: The pressure dependence of the ignition limits of acetylene-oxygen mixtures . West German Publisher, 1961.
- GW Jones, RE Kennedy: Effect of Pressure on Ignition Temperature of Acetylene and Acetylene-Air Mixtures. Report of Investigations, Bureau of Mines, Schwaz 1945.
Web links
- Entry on acetylenes in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
- Reactions of ethyne
Individual evidence
- ↑ a b c d e f g h i j Entry on acetylene in the GESTIS substance database of the IFA , accessed on March 13, 2019(JavaScript required) .
- ↑ a b c d e Entry on acetylene. In: Römpp Online . Georg Thieme Verlag, accessed on October 16, 2013.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ Entry on acetylenes in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 74-86-2 or acetylene ), accessed on September 15, 2019.
- ↑ a b c M. W. Chase, Jr .: NIST-JANAF Themochemical Tables. (= J. Phys. Chem. Ref. Data. Monograph 9). 4th edition. 1998.
- ↑ Renate Wahrig-Burfeind (Ed.): True. Illustrated dictionary of the German language . ADAC-Verlag, Munich 2004, ISBN 3-577-10051-6 , pp. 85 .
- ↑ a b c d e f g h P. Pässler, W. Hefner, K. Buckl, H. Meinass, A. Meiswinkel, H.-J. Wernicke, G. Ebersberg, R. Müller, J. Bässler, H. Behringer, D. Mayer: Acetylene. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2008. doi : 10.1002 / 14356007.a01_097.pub3 .
- ↑ M. Kutscheroff: About a new method of direct addition of water (hydration) to the hydrocarbons of the acetylene series. In: Reports of the German Chemical Society . Vol. 14, 1881, pp. 1540-1542. doi: 10.1002 / cber.188101401320 .
- ↑ History of the KUKA company .
- ↑ Acetylene Centers from Prof. Dr. JH Vogel 1901 .
- ↑ acetylene. In: Otto Lueger: Lexicon of the entire technology and its auxiliary sciences. Volume 1, Stuttgart / Leipzig 1904, pp. 56-61.
- ↑ Patent US4863493 : Applicant: Nichigo Acetylene Kabushiki.
- ↑ Patent JP2004148257 : Published May 27, 2004 , Applicants: Nichigo Acetylene & Taiyo Toyo Sanso.
- ↑ a b c d e W. Wiechmann: Official and information sheet of the Federal Institute for Materials Research and Testing (BAM). 3, 1987, p. 505.
- ↑ a b c d EIGA-IGC document 123/13 / D Practical guidelines for the safe handling of acetylene (PDF; 669 kB)
- ↑ D. Ambrose, R. Townsend: Vapor Pressure of Acetylene. In: Trans. Faraday Soc. 60, 1964, pp. 1025-1029; doi: 10.1039 / TF9646001025 .
- ↑ LV Gurvich, IV Veyts, CB Alcock: Thermodynamic Properties of Individual Substances. 4th edition. Vols. 1 and 2, Hemisphere, New York 1989.
- ↑ a b A. M. Clark, F. Din: Equilibria Between Solid, Liquid, and Gaseous Phases at Low Temperature binary systems acetylene - carbon dioxide, acetylene - ethylene and acetylene - ethane. In: Trans. Faraday Soc. 46, 1950, pp. 901-911. doi: 10.1039 / TF9504600901 .
- ↑ a b c d C. Tsonopoulos, D. Ambrose: Vapor-Liquid Critical Properties of Elements and Compounds. 6. Unsaturated Aliphatic Hydrocarbons. In: J. Chem. Eng. Data . 41, 1996, pp. 645-656. doi: 10.1021 / je9501999 .
- ^ W. Rimarski: Fire and explosion hazards with acetylene. In: Angew. Chem. 42, 1929, pp. 933-936. doi: 10.1002 / anie.19290423802 .
- ↑ a b c d e f E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ a b G. W. Jones, RE Kennedy: Effect of Pressure on Ignition Temperature of Acetylene and Acetylene-Air Mixtures. Report of Investigations, Bureau of Mines, Schwaz 1945. (digital library)
- ↑ Th. Schendler, H.-P. Schulze: Stability limit pressures of acetylene / gas mixtures. In: Chem. Ing. Techn. 62, 1990, pp. 41-43. doi: 10.1002 / cite.330620111 .
- ↑ D. Lietze, H. Pinkofsky, T. Schendler, H.-P. Schulze: Stability limit pressure of acetylene. In: Chem. Ing. Techn. 61, 1989, pp. 736-738. doi: 10.1002 / cite.330610915 .
- ↑ HB Sargent: Chem. Eng. 64, 1957, p. 250.
- ^ ME Sutherland, MW Wegert: Chem. Eng. Prog. 69, 1973, p. 48.
- ^ Danger of explosion in Graz: Cobra approached. ORF.at from July 7, 2014.
- ↑ BA Trofimov, SV Amosova: Divinyl Sulfide: Synthesis, Properties, and Applications. In: Sulfur reports. 3, 2007, p. 323, doi : 10.1080 / 01961778408082463 .
- ↑ Paula Yurkanis Bruice: Organic Chemistry. 5th edition. Pearson Education, 2007, ISBN 978-3-8273-7190-4 , p. 211.
- ^ Klaus Weissermel , Hans-Jürgen Arpe : Industrial organic chemistry: Significant preliminary and intermediate products. Wiley-VCH, 2007, ISBN 978-3-527-31540-6 , p. 132.
- ↑ Ernst Bartholomé: The development of the process for the production of acetylene by partial oxidation of hydrocarbons . In: Chemical Engineer Technology . tape 49 , no. 6 , June 1977, p. 459-463 , doi : 10.1002 / cite.330490602 ( PDF ).









![{\ displaystyle {\ ce {C2H2 + 2 [Ag (NH3) 2] + + 2NO3- -> Ag2C2 v + 2NH4NO3 + 2NH3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/22372ae9fa45aa9ff69cbff2ec797bcd58772adf)