Chloroprene rubber
Chloroprene rubber , also polychloroprene or chlorobutadiene rubber , is a synthetic rubber that is used , among other things, in automobile construction and for heat-insulating sportswear. In the German-speaking area it is known under the name neoprene . It is produced by polymerizing 2-chloro-1,3-butadiene ( chloroprene ). The abbreviation according to ISO 1043 (1975) for chloroprene rubber is CR .
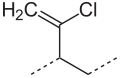
Structural formula
The double bonds can be found in both the trans and the cis positions ( cis , trans isomerism ). The trans : cis ratio is about 9: 1. The chlorine atoms are very inert and contribute to the stability and resistance of polychloroprene. The incorporation of monomers in the 1,2- or 3,4-position also occurs to a very small extent . The resulting structures are called 1,2 or 3,4 units and, depending on the polymerization temperature, occur in the order of one percent in the polymer chain :
Due to the much more reactive chlorine atom, the 1,2-unit is of crucial importance for the crosslinking ( vulcanization ) of the polymer.
history
The basic idea came from Julius Arthur Nieuwland , who worked with Wallace Hume Carothers from DuPont . In 1930, Arnold Collins , part of the Carothers team, polymerized polychloroprene for the first time under economically favorable conditions using the emulsion process. In 1932, the American company DuPont brought the polymer onto the market, initially under the name Duprene, and then in 1938 as Neoprene. In the following decades there were various improvements in terms of the manufacturing process and polymer properties:
- Copolymers with sulfur (Neoprene GN) for improved processability 1939 (DuPont);
- Mercaptan -regulated variants in the 1950s (DuPont) for improved heat resistance and solubility;
- Xanthate -modified chloroprene rubber in the eighties (Bayer AG) for improved properties of the vulcanized and reduced tendency to crystallize .
Manufacturing
Chloroprene rubber is produced on an industrial scale using the emulsion polymerization process. The resulting dispersion is precipitated by adding acid and subsequent cooling , dried and usually marketed in the form of chips for the processing industry. To prevent the chips from sticking together, they are powdered with talc . But the polymer dispersion itself is also used as an adhesive. B. the crystallizing types under the trade name Dispercoll® C.
Due to the regular structure and high proportion of 1,4- trans linkages in the monomer during polymerization, polychloroprene tends to crystallize more or less, which leads to hardening of the material some time after processing. This is desirable for adhesives, but less so for rubber articles. The tendency towards crystallization can be influenced accordingly in the desired direction through a suitable choice of the polymerization temperature and the co-monomers and through the use of regulators to set the molar mass .
Properties and uses
When dissolved in organic solvents, like the polymer dispersion itself, polychloroprene is also suitable for various adhesives due to its good resistance . Vulcanizates are characterized by chemical resistance, good resistance to embrittlement , weathering , ozone attack and flame retardancy.
- Good resistance to swelling in mineral oils with a high aniline point , fats , many refrigerants and water (with special compound structure).
- Medium swell resistance in mineral oils, low molecular weight aliphatic hydrocarbons ( light petrol , isooctane).
- Strongly swelling in aromatics, e.g. B. benzene , toluene , chlorinated hydrocarbons, esters , ethers , ketones .
- Thermal application range approx. −45 ° C to +100 ° C depending on the mixture composition (briefly up to 130 ° C).
Hoses, cable sheathing, extruded profiles, seals and drive belts based on chloroprene rubber can be found particularly in the automotive industry thanks to the favorable combinations of properties.
A known application, e.g. B. as a material for diving suits, is the foamed vulcanizate. By using chemical blowing agents , which release gases below the vulcanization temperature, a pressure-resistant foam or foam or sponge rubber with excellent insulating properties can be obtained.
Worldwide consumption of chloroprene rubber, including adhesives, is estimated at over 300,000 tons per year.
vulcanization
Unlike most other unsaturated elastomers, chloroprene rubber cannot be vulcanized with sulfur. Metal oxides such as zinc oxide (ZnO) and magnesium oxide (MgO) are usually used to vulcanize polychloroprene . For reasons of environmental protection to a limited extent, lead oxide can be used for improved water resistance . A typical vulcanization accelerator is ethylene thiourea (ETU also = E thylene T hio U rea) serving as a sulfur donor (sulfur donor) applies. The chemical structures that arise during the vulcanization of polychloroprene using ZnO and MgO in the presence of ETU are exclusively due to the reaction of allylically bound chlorine (see above: 1,2-unit), which is only present in a few percent in the polymer chain is. The majority of the vinylic bonded chlorine atoms (see above: 1,4-unit) practically do not react under the vulcanization conditions (approx. 160 ° C). For this reason, the crosslinking density of polychloroprene cannot be increased by adding more vulcanizing agents.
Ethylene thiourea (ETU) -based accelerators are the current industry standard for accelerating the vulcanization process. However, ETU has now been classified as possibly carcinogenic for humans by various European authorities. It is expected that the use of this substance will be restricted or banned by the EU in the near future. Small and medium-sized enterprises (SMEs) in the rubber industry will be particularly hard hit, especially given the increasing competition from Asian countries with less stringent health and safety regulations. A large consortium used the EU investment to find a safe substitute for ETU. As part of the SAFERUBBER project, scientists developed an environmentally friendly and inexpensive alternative. The "SRM102" additive had numerous important advantages. Chloroprene rubbers made with SRM102 have improved flow properties and are therefore much easier to fill into molds. This makes it possible to reduce both the amount of rubber used and the associated waste. In addition, the proportion of zinc oxide, a vulcanization activator, can be reduced.
Polychloroprene can be processed into polymer blends with various other polymers : with natural rubber (NR) or polybutadiene (BR) to reduce costs and improve low-temperature flexibility, with styrene-butadiene rubber (SBR) to reduce costs and reduce the tendency to crystallize, and with nitrile rubber (NBR) to improve oil resistance.
Foamed neoprene
Many small gas bubbles are evenly distributed in the foamed neoprene, which gives it excellent thermal insulation properties. This variant is best known for its cold protection suits for water sports ( diving suits , surf suits ). But also bottle coolers, sports bandages and protective covers of all kinds, sound insulation bearings for flights of stairs or other conditions are made from foamed neoprene.
For the use of sportswear, neoprene is produced in different thicknesses according to the desired thermal insulation . Thicker material insulates better, but is also less elastic and has a higher buoyancy .
As a rule, neoprene is laminated on both sides with textile fabric ( nylon or Lycra ) , which closes the surface and makes it less prone to damage. Smooth skin neoprene is only laminated on one side and has a closed, smooth rubber surface on one side. This material is suitable for sealing strips within neoprene clothing. In addition to the laminated suit versions, there are also uncovered ones. They are particularly elastic and, because of their tight fit, also water and heat insulating. The advantage lies particularly in the flexibility, which allows great freedom of movement. A disadvantage is their sensitivity to mechanical influences.
In the manufacture of neoprene clothing, the material is butt -glued together.
See also
Individual evidence
- ↑ a b RADO Gummi GmbH: CR. Retrieved May 26, 2020 .
- ↑ European Commission: CORDIS: Projects and Results: Getting started quickly and safely. Retrieved February 17, 2018 .
- ↑ A Safer Alternative Replacement for Thiourea Based Accelerators in the Production Process of Chloroprene Rubber ( English ) CORDIS. Retrieved February 8, 2019.
literature
- R. Musch, E. Rohde and H. Casselmann, Kautsch. Rubber, plastic 49 (1996) 340
- R. Musch, Hagg, The Polymeric Materials Encyclopedia, CRC Press, Inc. 1996