Copolymerization
The copolymerization is a special form a polymerization reaction . It differs from the “simple” polyreaction in that instead of one monomer (= homopolymerization), a mixture of two or more chemically different monomers is used. The product of the copolymerization is a copolymer .
General
In contrast to simple homopolymerization , in which only a single type of monomer is used, in copolymerization different monomers are reacted simultaneously or one after the other. The product of copolymerization is a copolymer that has different types of monomers in one molecule. Depending on the distribution of the different types of monomers in the polymer, a distinction is made between random, alternating, block and graft copolymers.
Copolymerization makes a variety of materials accessible. By matching the type and molar ratios of the monomers in the polymer, many properties can be varied over a wide range. Thermoplastic elastomers are an important class . At elevated temperatures, these become thermoplastics and, like them, can be easily processed. After cooling, they show (rubber) elastic properties again. Also plasticization (internal plasticization) can be reached by copolymerization. An external plasticizer is then no longer required.
Reaction kinetics
For the process control of the synthesis it is important to know the composition of the copolymer. The monomers must be used accordingly. If we first consider the synthesis of a copolymer from two monomers (the model applies accordingly to higher systems). In principle, four individual reactions at the chain end of a macromolecule are possible for its propagation:
From the four rate constants one now creates quotients, which are also called copolymerization parameters:
- and
The parameters indicate the probability with which the monomer M or M is attached to a chain end which carries an M or M group.
Is z. B. , the monomer 2 is preferably attached to the chain end 1. For it is exactly the opposite. If both parameters are equal to one, then none of the four reactions is preferred and a purely randomly distributed copolymer results. From the law of speed
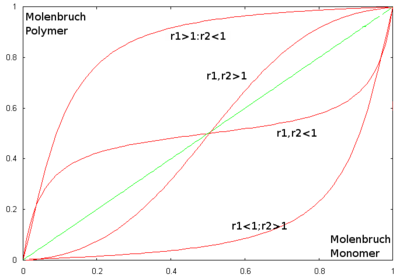
the mole fractions in the polymer can be calculated, since the quotient of rate constants is an equilibrium constant . If the molar fraction of a monomer species in the reaction mixture is plotted against its molar fraction in the polymer, the copolymerization diagram shown is obtained. The intersection of the curves with the bisector (here green) represents the azeotropic point . If a reaction is carried out under these conditions, the mole fraction of a species in the reaction mixture is just as large as in the resulting polymer. In other cases there is a shift in the mole fraction during the reaction. This is known as drift .
Reactivity of the monomers
The reactivity of the monomers and thus the incorporation behavior of the monomer components into the copolymer are fundamentally dominated by two factors.
- Resonance effects
- Polarity effects
The reactivity of a monomer can be described by its kinetic activity. The copolymerization behavior is determined by the copolymerization parameter r and is consequently a function of the reactivities of the individual monomers.
Resonance effects
The reactivity of a vinyl monomer is determined by the structure of its substituent. Resonance-stabilized vinyl groups have an increased activity of the monomer towards the radical, depending on the strength of the stabilization. Highly resonance-stabilized vinyl monomers are reactive monomers and at the same time inert radicals. As a result, if the monomer combination is unsuitable (e.g. styrene / vinyl acetate), homopolymerization occurs because the reactivity of the two components is too different (r 1 = 55, r 2 = 0.01).
Polarity effects
The polarization of the vinylic double bond also has a major influence on the copolymerization behavior. Electron-withdrawing substituents (EWG's) lower the electron density of the double bond, whereas electron donors increase it. The electron-withdrawing groups include molecular structures with a pronounced -I or -M effect , such as esters, ketones or cyanides. The electron donors, those with + I or + M properties, can be assigned alkyls or ethers. A monomer combination of monomers of different polarity shows a tendency towards an alternating monomer sequence in the copolymer. If the polarities are similar to one another, a statistical sequence is carried out.
Qe scheme
A semi-empirical method of assigning reactivities Q and polarities e to monomer compounds was described by Alfrey and Price. With this method, reactivity ratios can be calculated based on these two parameters alone. The graphical plot of the reactivities of various monomers in the Qe coordinate system is also known as the Qe scheme.
The rate constant of a polymer growth step is described by the Arrhenius equation . The factors reactivity Q and polarity e are integrated into the activation energy, whereby the following relationship can be given.
Here p 1 is the reactivity of the active chain end, q 1 that of the monomer and e 1 and e 2 are the polarities of active chain end and monomer. The reactivities can be included in the frequency factor A 12 so that the following relationship is obtained.
The parameters Q (P) and e are determined from experimental results (determined r 1 , r 2 values) and set in relation to the standard styrene, which has been assigned the values Q = 1 and e = −0.8 at random.
literature
- B. Tieke: Macromolecular Chemistry. Wiley-VCH, 2005, ISBN 3-527-31379-6 .