Mesomerism
In chemistry, mesomerism (also resonance or resonance structure ) describes the phenomenon that the bonding relationships in some molecules or polyatomic ions cannot be represented by a single structural formula , but only by several limit formulas . None of these limit formulas adequately describes the bonding conditions and thus the distribution of the electrons . The actual electron distribution in the molecule or ion lies between the electron distributions given by the limit formulas. This is represented by the mesomeric arrow ( resonance arrow ) with the symbol ↔ . The mesomeric arrow should not be confused with the double arrow equilibrium arrow ⇌, which indicates a chemical equilibrium. The term mesomerism was introduced in 1933 by Christopher Kelk Ingold .
The real state of a molecule, i.e. the intermediate state between the boundary structures, is called the mesomeric state .
The energy difference between the boundary structures and the actual mesomeric state, which in many cases can be estimated, is called the mesomeric energy or resonance energy . The more mesomeric boundary structures a molecule or ion has, the more stable it is. Within the framework of the valence structure theory (VB theory), the natural energies of the individual boundary structures and their weight (i.e. their share in the overall wave function) can be calculated.
Example benzene
| Benzene (C 6 H 6 ) | |
| mesomeric boundary structures | |

|
|
| delocalized double bonds | usual representation |
 |
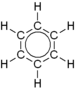
|
| Mesomeric energy | |

|
|
An example of a mesomeric compound is benzene (see figure). Other aromatics are also mesomeric compounds.
According to the octet rule , those molecules are particularly stable in which each atom is surrounded by eight valence electrons . Two structural formulas can be drawn up for benzene in which this is the case (mesomeric boundary structures).
That neither of the two limiting formulas of benzene is correct can be deduced from the bond lengths of the bonds between the carbon atoms. The carbon atoms linked to one another by double bonds would have to be closer together than those which are linked to one another only by a single atomic bond . However, this is not the case. The bond lengths between the carbon atoms are uniformly 139 pm .
In the benzene ring, each carbon atom has four valence electrons , two of which connect the atom to the neighboring carbon atoms. An electron binds the associated hydrogen atom. The remaining six π - electron yield three π- formal bonds , as expressed in the structural formula with three double bonds. In the orbital model that is valid today , the six π electrons form a delocalized charge cloud (delocalized 6 π electron system) above and below the level of the carbon ring ( multi-center bond ).
This results in an energy state reduced by 151 kJ / mol , which increases the binding energy by the same amount, which results in greater stability compared to the hypothetical limiting formulas ( cyclohexatriene ) with three isolated double bonds. This energy difference is called the mesomeric or resonance energy and results from the difference in the hydrogenation energies of the hypothetical cyclohexatriene and benzene.
The same value results from the difference in the combustion energies of the two compounds.
However, another reference substance can also be used for the mesomeric energy. In the case of a comparison of the hydrogenation energies or combustion energies of benzene with the corresponding linear molecule (hexatriene), a somewhat lower value results. This resonance energy is called "Dewar Resonance Energy (DRE)".
Salts of carboxylic acids and vinylogous carboxylic acids
A very similar case of delocalized electrons is found in the salts of carboxylic acids such as formic and acetic acid ; here, too, this leads to the mesomerism stabilization of the carboxylate anion (formic acid: formate anion; acetic acid: acetate anion). By inserting one or more C = C double bonds, salts of vinylogous carboxylic acids are obtained. The mesomerism is not lost. Mesomeric-stabilized carboxylate anion (top) and vinylogous carboxylate anion (bottom):
In the upper case (carboxylate anion), the mesomerism can be indicated by the following notation:
Examples of vinylogous carboxylic acids are ascorbic acid (vitamin C) and vulpinic acid .
See also
Individual evidence
- ↑ Entry on mesomerism . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03845 Version: 2.1.5.
- ↑ Entry on resonance . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05326 Version: 2.1.5.
- ^ Sason S. Shaik, Philippe C. Hiberty: A Chemist's Guide to Valence Bond Theory . John Wiley & Sons, 2007, ISBN 978-0-470-19258-0 .

