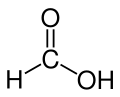
Formic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Formic acid | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | CH 2 O 2 | |||||||||||||||||||||
| Brief description |
colorless, pungent smelling liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 46.03 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.22 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
8 ° C |
|||||||||||||||||||||
| boiling point |
101 ° C (decomposition) |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
3.77 |
|||||||||||||||||||||
| solubility |
miscible with water, ethanol , glycerine and diethyl ether |
|||||||||||||||||||||
| Refractive index |
1.3714 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−425.0 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Formic acid (according to the nomenclature of the IUPAC formic acid lat. Acidum formicum of formica , ant ') is a colorless, corrosive and water-soluble liquid, which is used in nature in many living beings for defense purposes. With the semi-structural formula HCOOH, it is the simplest carboxylic acid and the shortest-chain alkanoic acid ; the carboxy group (–COOH) determines its properties particularly strongly. The carbon atom has an oxidation state of +2. It can therefore act as a hydride transfer analogous to the carbonyl compounds , hence its reducing effect. The salts of formic acid are called formates (systematically also methanoates ) and have the semi- structural formula (HCOO) n M, where n corresponds to the valence of the metal ion. Examples of formates are sodium formate , HCOONa, and aluminum formate , (HCOO) 3 Al. The esters of formic acid are also called formates.
history
In the early 15th century, some alchemists and naturalists observed that certain ants (species belonging to the group of scale ants ) secrete an acidic liquid.
The English naturalist John Ray was the first to isolate formic acid in 1671 by distilling large numbers of ants . In 1792 the doctor Christoph Girtanner wrote the following text about the extraction of formic acid:
“The formic acid is obtained by distillation from the large ants ( Formica rufa ). Ants are distilled over a gentle fire, and the formic acid is obtained in the template. It makes up about half the weight of the ants. Or you wash the ants in cold water, then lay them on a cloth and pour boiling water over them. If you squeeze the ants gently, the acid becomes stronger. To purify the acid, it is subjected to repeated distillation, and to concentrate it, it is allowed to freeze. "
The French chemist Joseph Louis Gay-Lussac was the first to synthesize formic acid from hydrogen cyanide . In 1855 another French chemist, Marcellin Berthelot , invented the synthesis from carbon monoxide , which is still used today. For a long time, formic acid was of little technical importance. In the late 1960s, significant amounts of formic acid were produced as a by-product in the synthesis of acetic acid . Only later was formic acid used on a larger scale. It was now no longer just obtained as a by-product, but specifically produced synthetically.
Occurrence
Formic acid is widespread in nature. It is used by many plant and animal species, especially by voices , as a component of poison mixtures for defense and attack purposes.
Formic acid is a natural component of bee honey ; Depending on the variety, 1 kilogram of honey contains 50 to over 1000 milligrams. Formic acid is also a component of tobacco smoke .
The caterpillars of the great fork tail ( Cerura vinula ) - a species of butterfly - as well as some species of ants (members of the subfamily Formicinae ) spray a liquid containing formic acid as a defense. Some species of ground beetles , scorpions and bees use secretions containing formic acid for both defense and attack purposes. In some species of jellyfish , formic acid is part of the poison in the nettle capsules.
In the stinging hairs of the nettles there is a nettle poison that contains, among other things, formic acid and sodium formate.
In the human body formic acid formed in addition to formaldehyde in the metabolism of methanol . Formic acid is easily biodegradable.
Traces of formic acid in space were detected spectroscopically. In the shell ( coma ) of the comet Hale-Bopp , in 2000 by D. Bockelée-Morvan and others, 0.09% formic acid (based on water = 100%) was used for the first time in addition to other organic compounds such as hydrocyanic acid, acetonitrile, methanol or methyl formate. found. There are two theories for the formation of these compounds: either the compounds were originally located in the nucleus of the comet and flow out of there, or they arise from gas phase reactions in the coma. According to simulations, however, the former is likely due to the distributions.
Extraction, manufacture
The historical isolation of formic acid from dead ants is no longer carried out today. In the chemical industry, formic acid is usually produced using the process invented by Marcellin Berthelot in 1855. The synthesis is divided into two process steps:
- Sodium hydroxide reacts with carbon monoxide at about 6–8 bar and 130 ° C to form sodium formate .
- Sodium formate is reacted with sulfuric acid to form formic acid and sodium sulfate .
Formic acid is also produced from methanol with the help of carbon monoxide , among other things . Here, too, two procedural steps are carried out. Methyl formate is produced as an intermediate. In the end, methanol is recovered, which can be used again as a starting product for this synthesis :
- At 80 ° C and 40 bar, methanol reacts with carbon monoxide to form methyl formate .
- Formic acid methyl ester reacts with water to form formic acid and methanol.
Because the hydrolysis of methyl formate would use a lot of water, some formic acid manufacturers use an indirect process with ammonia , which in turn requires two process steps. However, this indirect process has problems because the by-product ammonium sulfate is partially released:
- Formic acid methyl ester reacts with ammonia to form formamide and methanol.
- Formamide reacts with sulfuric acid to form formic acid and ammonium sulfate .
- Hydrolysis of chloroform (trichloride of orthoformic acid) with KOH
Because of this problem, the manufacturers have developed a new process of direct hydrolysis, in which the formic acid can be separated from the large amounts of water in an energy-efficient manner ( liquid extraction ).
Formic acid occurs as a by-product in the production of acetic acid from light gasoline or n -butane and can also be produced with the help of hydrocyanic acid. There is a second method for production from methanol. Here, methanol is converted to formaldehyde and formic acid. However, these three processes are of little technical importance.
Biosynthetically, formic acid is released from glycine or serine and tetrahydrofolic acid via formyltetrahydrofolic acid in the poison glands of ants.
properties
Physical Properties
Formic acid is a relatively unstable, colorless, clear and highly volatile liquid. At 8 ° C it solidifies to a colorless solid; it boils at 100.7 ° C. Melting and boiling points are significantly higher than those of organic compounds with similar molar masses ( e.g. propane ), since hydrogen bonds between the individual molecules also have to be broken when they melt and boil . Some of these persist in the gaseous state, which is why formic acid deviates significantly from the behavior of an ideal gas . It forms an azeotrope with water .
Formic acid has a density of 1.22 g · cm −3 at 20 ° C. To melt the formic acid, 12.7 kJ / mol are required, and 22.7 kJ / mol to evaporate. The triple point is 8.3 ° C and 0.0236 bar.
Formic acid has a strong and pungent odor. The odor threshold is 1 ml / m 3 . Formic acid can be mixed with water, ethanol and glycol in any ratio. It is also soluble in most other polar organic substances, but only in small amounts in hydrocarbons .
The acid constant (p K s value) is 3.77. It is the strongest unsubstituted monocarboxylic acid . For comparison: hydrochloric acid has a p K s value of −7, sulfuric acid of −3.
The detection of formic acid vapors (e.g. to determine the concentration in the workplace ) can be done with the help of gas detection devices . Otherwise, the formic acid is detected through its reducing effect, usually because it can reduce an ammoniacal silver nitrate solution to silver .
Thermodynamic properties
The standard enthalpy of formation Δ f H 0 liquid is −424.72 kJ mol −1 , Δ f H 0 gas is −378.6 kJ mol −1 .
The standard entropy S 0 liquid is 128.95 J mol −1 K −1 , S 0 gas 248.7 J mol −1 K −1 .
The heat capacity of the liquid is given as 99.04 J mol −1 K −1 (25 ° C), that of the gas as 45.7 J mol −1 K −1 (25 ° C).
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 2.00121, B = 515.000 K and C = −139.408 K. in the temperature range from 273.6 K to 307.3 K.
Chemical properties
In the presence of oxygen, formic acid burns to carbon dioxide and water. Formic acid is a strong reducing agent because it is also an aldehyde (hydroxy formaldehyde ).
- Formic acid burns with oxygen to form carbon dioxide and water.
- Formic acid decomposes in the presence of conc. Sulfuric acid to water and carbon monoxide .
- At higher temperatures and in the presence of a catalyst ( platinum , palladium ) it breaks down to carbon dioxide and hydrogen .
Formic acid reacts with metals to form metal formates and hydrogen:
Sodium reacts with formic acid to form sodium formate, forming hydrogen .
- Formic acid reduces silver ions in the alkaline.
Formic acid reacts with alcohols in the presence of a catalyst (usually sulfuric acid) to form water and alkyl formates.
- Formic acid reacts with methanol to form water and methyl formate.
Safety-related parameters
Formic acid is considered a flammable liquid. Flammable vapor-air mixtures can form above the flash point . The compound has a flash point of 45 ° C. The explosion range is between 10 % by volume (190 g / m 3 ) as the lower explosion limit (LEL) and 45.5% by volume (865 g / m 3 ) as the upper explosion limit (UEL). With a standard gap width of 1.76 mm, it is assigned to explosion group IIA. The ignition temperature is 520 ° C. The substance therefore falls into temperature class T1. The electrical conductivity is low at 6.08 · 10 −3 S · m −1 .
use
Until 1998, formic acid was used as a preservative in fish, fruit and vegetable products under the E number E236 , but has since then - in contrast to Switzerland - no longer been approved as a food additive in the EU . The related substances sodium and calcium formate are also no longer permitted as food additives (E237 and E238). In medicine it is used as an anti-inflammatory drug, as well as for the treatment of vulgar warts . A ready-to-use solution containing formic acid is applied to the wart. In the textile and leather industry they are used for pickling and impregnating. Sometimes it is also used as a disinfectant (also in acidic cleaning agents). According to the import regulations of the EU it is z. B. used for certain goods from other EU countries to prevent the spread of animal diseases . It also kills bacteria well. In the chemical industry it is used to neutralize alkaline reaction mixtures. In electronics production, formic acid is used as a reducing agent in the soldering process . It is used industrially for the decalcification of cooling water systems, since the resulting wastewater only contains the harmless calcium formate with a low COD value.
Beekeepers usually use them in a 60% concentration, in strictly indicated exceptional cases up to a maximum of 85% concentration in an aqueous solution to treat the bees against the Varroa mite . In genetics , formic acid can be used in conjunction with the enzyme AP endonuclease to create insertion mutants at random , known as in vitro mutagenesis. In the plastics industry it is used to glue polyamide plastics .
Concentrated formic acid is used to clean raw gemstones because it attacks limestone and other impurities heavily, exposing the gemstone without damaging it. This cleaning process should only be used with acid-resistant gemstones.
Scientists at the Leibniz Institute for Catalysis have experimentally achieved the catalytic release of hydrogen from formic acid even at room temperature. This hydrogen could e.g. B. converted to electricity in fuel cells . This possibility is to be used for small-scale storage of energy. The direct formation reaction of formic acid from hydrogen and carbon dioxide is thermodynamically very limited and the efficiency of the corresponding processes is therefore rather low.
Health hazards
Formic acid can be broken down by the body. Direct contact with formic acid or concentrated vapors is irritating to the respiratory tract and eyes. At concentrations above ten percent, skin contact leads to severe burns and blisters. With long-term exposure, it can cause skin allergies, in extremely high doses as well as chemical burns and necrosis of the oral and pharyngeal mucosa, the esophagus and the gastrointestinal tract, acidosis , unconsciousness, drop in blood pressure, damage to the blood, liver and kidneys as well as pneumonia and damage to the heart cause.
The decomposition of formic acid can produce the breath toxin carbon monoxide . The acid must be stored in a well-ventilated, cool place. Containers in which formic acid is stored must also be closed with a pressure equalization screw connection, since the gases formed during decomposition can generate excess pressure.
Individual evidence
- ↑ entry to FORMIC ACID in CosIng database of the European Commission, accessed on February 25 2020th
- ↑ a b c d e f g h i j k l m n o Entry on formic acid in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF; 78 kB).
- ↑ Entry on formic acid. In: Römpp Online . Georg Thieme Verlag, accessed on May 24, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-262.
- ↑ Entry on Formic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 64-18-6 or formic acid ), accessed on September 14, 2019.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ Christoph Girtanner: Beginnings of anti-inflammatory chemistry. 1st edition, 1792, p. 389 ( limited preview in the Google book search).
- ↑ Beekeeping Association Tempelhof: Alternative Varroa Control ( Memento from January 3, 2015 in the Internet Archive ).
- ↑ D. Bockelée-Morvan et al .: New molecules found in comet C / 1995 O1 (Hale-Bopp): Investigating the link between cometary and interstellar material. In: Astronomy and Astrophysics , 2000, 353, pp. 1101-1114, bibcode : 2000A & A ... 353.1101B .
- ↑ SD Rodgers, SD Charnley: Organic synthesis in the coma of comet Hale-Bopp? In: Monthly Notices of the Royal Astronomical Society , 2000, 320, 4, pp. L61 – L64, bibcode : 2001MNRAS.320L..61R .
- ↑ Arnold Willmes: Taschenbuch Chemical Substances , Harri Deutsch, Frankfurt (M.), 2007, ISBN 978-3-8171-1787-1 .
- ↑ Abraham Hefetz, Murray S. Blum: Biosynthesis of formic acid by the poison glands of ants formicine . In: Biochimica et Biophysica Acta - General Subjects . tape 543 , no. 4 , 1978, p. 484-496 , doi : 10.1016 / 0304-4165 (78) 90303-3 .
- ↑ a b c d Entry on formic acid . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed July 20, 2012.
- ↑ Technical rule for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 .
- ↑ Gita Faghihi, Anahita Vali, Mohammadreza Radan, Golamreza Eslamieh, Shadi Tajammoli: A double-blind, randomized trial of local formic acid puncture technique in the treatment of common warts . In: Skinmed . tape 8 , no. 2 , 2016, ISSN 1540-9740 , p. 70-71 , PMID 20527136 ( skinmedjournal.com [PDF]). A double-blind, randomized trial of local formic acid puncture technique in the treatment of common warts ( Memento from November 12, 2015 in the Internet Archive )
- ↑ RM Bhat, K. Vidya, G. Kamath: Topical formic acid puncture technique for the treatment of common warts . In: International Journal of Dermatology . tape 40 , no. 6 , 2001, p. 415-419 , PMID 11589750 .
- ↑ FAZ.net: Foreign deployment material from Afghanistan lands in Emden , August 10, 2013.
- ↑ Apiculture / bee health / varroosis / information materials. Service Center for the Rural Rhine Palatinate Region, accessed on October 24, 2018 .
- ^ Electricity from formic acid. Reported to heise.de on May 7, 2008.
- ↑ Roland Knauer: Formic acid as an intermediate storage for hydrogen. The Leibniz Institute for Catalysis is developing a storage system for clean energy . In: Leibniz . Issue 3/2010, pp. 18-19, Leibniz Association, Bonn 2010.
- ↑ Jan Oliver Löfken: Formic acid as hydrogen storage. A new iron catalyst releases the energy-rich gas even under mild conditions . Report at weltderphysik.de from September 23, 2011.
- ↑ Séverine Moret, Paul J. Dyson & Gábor Laurenczy: Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media , Nature Communications 5, Article number: 4017, doi: 10.1038 / ncomms5017 .
- ↑ Irma Schmidt, Karsten Müller, Wolfgang Arlt : Evaluation of Formic-Acid-Based Hydrogen Storage Technologies , Energy & Fuels , 2014, 58, 540-6544, doi: 10.1021 / ef501802r .
literature
- Formic acid, formates, diglycol bis-chloroformate. VCH, Weinheim 1992, ISBN 3-527-28529-6 .
- Selected CHO radicals. Formic acid. Acetic acid. Oxalic acid (Gmelin Handbook of Inorganic and Organometallic Chemistry - 8th edition ELEM C TL C LFG 4). Springer, Berlin 1975, ISBN 3-540-93283-6 .
- Gundula Jänsch-Kaiser and Dieter Behrens: Formic acid and alkaline earth hydroxides. DECHEMA Society for Chemical Technology and Biotechnology e. V., ISBN 3-926959-00-2 .
Web links
- Medicine and Dangers ( Memento from January 11, 2006 in the Internet Archive )
- Properties, presentation and use
- Ingrid Rieck: Novel process for generating hydrogen. University of Rostock, press release from September 23, 2011 at the Informationsdienst Wissenschaft (idw-online.de), accessed on August 24, 2015.




















