Caproic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
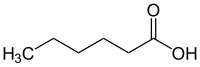
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Caproic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O 2 | |||||||||||||||
| Brief description |
oily, colorless to pale yellow liquid with a pungent, sweaty odor of goat |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 116.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.9212 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
−4 ° C |
|||||||||||||||
| boiling point |
206 ° C |
|||||||||||||||
| Vapor pressure |
30 Pa (20 ° C) |
|||||||||||||||
| pK s value |
4.85 (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4163 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Caproic acid ( n -hexanoic acid) is a saturated fatty or carboxylic acid that is derived from n -hexane . As with caprylic acid and capric acid, the name is derived from the Latin capra for goat, which, like the historical trivial name “goat acid”, indicates the acid's characteristic odor.
It is a colorless, oily, foul-smelling liquid at room temperature. It occurs chemically bound to 2–3 percent in triglycerides of the milk fat in milk, as well as in coconut oil . It also comes esterified in various seed oils and essential oils ; Spruce needle oil , lavender oil , lemongrass oil and also in fruits e.g. B. strawberry, raspberry , as well as in beer and coffee. Like many other carboxylic acids, caproic acid is used to synthesize fruit flavors through esterification . Their salts and esters are called capronates and hexanoates . In the fatty acid nomenclature it has the designation 6: 0.
Web links
Individual evidence
- ↑ Chevreul: About the causes of the differences in soap with regard to hardness, softness and smell, and about a new group of organic acids. In: Polytechnisches Journal . 11, 1823, p. 428.
- ↑ Rudolph Böttger : Tabular overview of the specific weights of the body . 1837, p. 26 ( full text / preview in Google book search).
- ^ Otto Ule , Karl Müller : The nature: newspaper for the dissemination of scientific knowledge ... Volume 8, Halle, 1859, p. 171, limited preview in the Google book search.
- ↑ a b c d e f Entry on caproic acid in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Entry on hexanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 97th edition. (Internet version: 2016), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-298.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-46.
- ↑ Claudia Synowietz (Ed.): Paperback for chemists and physicists . founded by Jean d'Ans, Ellen Lax. 4th edition. Volume II: Organic Compounds . Springer, Berlin 1983, ISBN 3-540-12263-X .
- ↑ Walter Karrer : Constitution and occurrence of organic plant substances. Springer, 1958, ISBN 978-3-0348-6808-2 (reprint), p. 289.
- ↑ Hexanoic acid at PlantFA Database, accessed November 7, 2017.
- ↑ J. Schormüller : The components of food. Springer, 1965, ISBN 978-3-642-46012-8 , p. 768.

