Common name (chemistry)
Trivial names are names for chemical compounds that do not comply with the systematic nomenclature rules of chemistry and therefore only in rare cases and then only partially provide information about the composition and structure of the compound, for example Michler's ketone , where the partial structure is called ketone . Trivial names mostly come from before the introduction of the nomenclature or are used for the sake of simplicity when the systematic name is very complicated. In some cases (for example with heterocycles , biomolecules or condensed polycyclic hydrocarbons ), trivial names have also entered today's systematic nomenclature as components. In other cases, too, trivial names are often used on an equal footing with the IUPAC names.
For many organic substances, almost only trivial names are used, since it is becoming increasingly difficult to give a correct IUPAC name at all for complex organic substances. Such IUPAC names are often very long and complex; they can hinder technical communication instead of making it easier.
The substances listed in the following lists represent an exemplary selection. They do not contain any trivial names for mixtures of substances and minerals.
Inorganic substances
Solid substances
liquids
Almost without exception, the substances mentioned here are aqueous solutions.
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Barite water | Barium hydroxide | Ba (OH) 2 |
| Boron water | Orthoboric acid | H 3 BO 3 |
| Caro's acid | Peroxomonosulfuric acid | H 2 SO 5 |
| Eau de Javel, Javel water | Potassium hypochlorite | KOCl (aq) |
| Eau de Labarraque | Sodium hypochlorite | NaOCl (aq) |
| Hydrofluoric acid | Hydrofluoric acid | HF |
| Milk of lime | Calcium hydroxide | Ca (OH) 2 |
| Potassium hydroxide | Potassium hydroxide | KOH |
| Lime water | Calcium hydroxide | Ca (OH) 2 |
| Magnesia milk | Magnesium hydroxide | Mg (OH) 2 |
| Caustic soda | Sodium hydroxide | NaOH |
| Oleum | sulfuric acid | H 2 SO 4 |
| Pyrosulfuric acid | Disulfuric acid | H 2 S 2 O 7 |
| Ammonia | ammonia | NH 3 |
| hydrochloric acid | Hydrochloric acid | HCl |
| Separating water | nitric acid | ENT 3 |
| Heavy water | Deuterium oxide | D 2 O |
| Heavy water | Tritium oxide | T 2 O |
| Theater blood | Iron thiocyanate | Fe (SCN) 3 |
| Vitriol oil | sulfuric acid | H 2 SO 4 |
| water | Dihydrogen oxide , oxidan | H 2 O |
Gases
| Common name | IUPAC name | chemical formula |
|---|---|---|
| ammonia | Nitrogen trihydride | NH 3 |
| Prussic acid | Hydrogen cyanide | HCN |
| steam | Dihydrogen oxide , oxidan (gaseous) | H 2 O |
| Pop acid | Formonitrile oxide | HCNO |
| Carbon dioxide | carbon dioxide | CO 2 |
| Carbon monoxide | Carbon monoxide | CO |
| Laughing gas | Nitrous oxide | N 2 O |
| ozone | Tri-oxygen | O 3 |
| Sulfane | Hydrogen sulfide | H 2 S |
Organic substance
Alkanes / alkenes / alkynes / haloalkanes and alkenes
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Carbon tetrachloride | Carbon tetrachloride | CCl 4 |
| chloroform | Trichloromethane | CHCl 3 |
| Iodoform | Triiodomethane | CHI 3 |
| Per (chlorine) | Tetrachlorethylene | C 2 Cl 4 |
| Tri (chlorine) | Trichlorethylene | C 2 HCl 3 |
| Freon 12 | Dichlorodifluoromethane | CCl 2 F 2 |
| Ethylene | Ethene | C 2 H 4 |
| acetylene | Ethine | C 2 H 2 |
Alcohols
| Common name | IUPAC name | chemical formula |
|---|---|---|
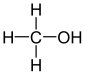
| Alcohol , drinking alcohol , alcohol, primate spirit , alcohol | Ethanol | |
| Amyl alcohol | Pentan-1-ol | |
| Carbinol , wood alcohol , wood spirit | Methanol | |
| Ethylene glycol , glycol | Ethane-1,2-diol | |
| Glycerin , glycerol | Propane-1,2,3-triol | |
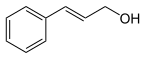
| Cinnamon alcohol | 3-phenyl-2-propenol |
Phenols
| Common name | IUPAC name | chemical formula |
|---|---|---|
| phenol | Hydroxybenzene | |
| Catechol | 1,2-dihydroxybenzene | |
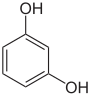
| Resorcinol | 1,3-dihydroxybenzene | |
| Hydroquinone | 1,4-dihydroxybenzene | |
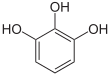
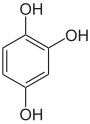
| Pyrogallol | 1,2,3-trihydroxybenzene | |
| Hydroxyhydroquinone | 1,2,4-trihydroxybenzene | |
| Phloroglucine | 1,3,5-trihydroxybenzene |
Aldehydes
| Common name | IUPAC name | chemical formula |
|---|---|---|
| acetaldehyde | Ethanal | |
|
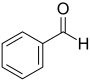
Benzaldehyde bitter almond oil |
Phenylmethanal | |
| Butyraldehyde | Butanal | |
| formaldehyde | Methanal | |
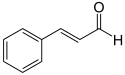
| Cinnamaldehyde | 3-phenyl-2-propenal |
Ketones / ethers
| Common name | IUPAC name | chemical formula |
|---|---|---|
| acetone | 2-propanone / dimethyl ketone | |
| Ether | Diethyl ether | |
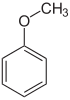
| Anisole | Methoxybenzene | |
| Dioxane | 1-4-dioxacyclohexane |
Carbonic acid derivatives
| Common name | IUPAC name | chemical formula |
|---|---|---|
| urea | Carbonic acid diamide | (NH 2 ) 2 CO |
| Phosgene | Carbonic acid dichloride | Cl 2 CO |
Organic acids
Monocarboxylic acids
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Formic acid | Methanoic acid | HCOOH |
| acetic acid | Ethanoic acid | CH 3 COOH |
| Propionic acid | Propanoic acid | C 2 H 5 COOH |
| Butyric acid | Butanoic acid | C 3 H 7 COOH |
| Valeric acid | Pentanoic acid | C 4 H 9 COOH |
| Caproic acid | Hexanoic acid | C 5 H 11 COOH |
| Enanthic acid / pimelic acid | Heptanoic acid | C 6 H 13 COOH |
| Caprylic acid | Octanoic acid | C 7 H 15 COOH |
| Pelargonic acid | Nonanoic acid | C 8 H 17 COOH |
| Capric acid | Decanoic acid | C 9 H 19 COOH |
| Lauric acid | Dodecanoic acid | C 11 H 23 COOH |
| Myristic acid | Tetradecanoic acid | C 13 H 27 COOH |
| Palmitic acid | Hexadecanoic acid | C 15 H 31 COOH |
| Margaric acid | Heptadecanoic acid | C 16 H 33 COOH |
| Stearic acid | Octadecanoic acid | C 17 H 35 COOH |
| Arachidic acid | Eicosanoic acid | C 19 H 39 COOH |
| Behenic acid | Docosanoic acid | C 21 H 43 COOH |
| Lignoceric acid | Tetracosanoic acid | C 23 H 47 COOH |
| Cerotic acid | Hexacosanoic acid | C 25 H 51 COOH |
| Montanic acid | Octacosanoic acid | C 27 H 53 COOH |
| Melissic acid | Triacontanoic acid | C 29 H 57 COOH |
Other carboxylic acids
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Acetoacetic acid | 3-oxobutanoic acid | |
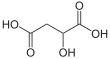
| Malic acid | 2-hydroxybutane-1,4-diacid | |
| Ascorbic acid , vitamin C. | ( R ) -5 - (( S ) -1,2-dihydroxyethyl) -3,4-dihydroxy-5 H -furan-2-one | |
| Benzilic acid | Hydroxydiphenylethanoic acid | |
| Succinic acid | 1,4-butanedioic acid | |
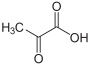
| Pyruvic acid | 2-oxopropanoic acid | |
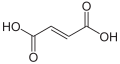
| Fumaric acid | ( E ) -2-butenedioic acid | |
| Glycolic acid | 2-hydroxyethanoic acid | |
| Glyoxylic acid | Ethanal acid | |
| Maleic acid | ( Z ) -2-butenedioic acid |

|
| Malonic acid | 1,3-propanedioic acid | |
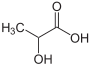
| Lactic acid | 2-hydroxypropanoic acid | |
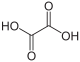
| Oxalic acid | Ethanedioic acid | |
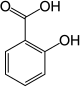
| Salicylic acid | 2-hydroxybenzoic acid | |
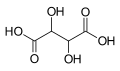
| Tartaric acid | 2,3-dihydroxybutanedioic acid | |
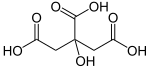
| citric acid | 2-hydroxypropane-1,2,3-tricarboxylic acid | |
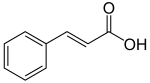
| Cinnamic acid | ( E ) -3-phenylpropenoic acid | |
| Sugar acid | Glucaric acid | |
| see also: List of acids | ||
Ester
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Amyl acetate | Pentyl ethanoate | CH 3 COOC 5 H 11 |
| Pear ether | Pentyl ethanoate | CH 3 COOC 5 H 11 |
| Vinegar ether | Ethyl ethanoate | CH 3 COOC 2 H 5 |
| Nitroglycerin | Glycerol trinitrate | C 3 H 5 (ONO 2 ) 3 |
Amines
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Cadaverine | 1,5-diaminopentane |
|
| Putrescine | 1,4-diaminobutane |
|
Multifunctional connections
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Acetylsalicylic acid | 2- (acetyloxy) benzoic acid |

|
| Paracetamol | N- (4-hydroxyphenyl) acetamide |
|
Organic salts
| Common name | IUPAC name | chemical formula |
|---|---|---|
| Lead sugar | Lead (II) acetate | Pb (CH 3 COO) 2 |
| Emetic tartar | Potassium antimonyl tartrate | K [C 4 H 2 O 6 Sb (OH) 2 ] • 1/2 H 2 O |
| Acetic clay | Aluminum diacetate | Al (CH 3 COO) 2 OH |
| Verdigris | Copper (II) acetate | basic copper acetate |
| Meerwein salt (imprecise) | Trimethyloxonium tetrafluoroborate |

|
| Rochelle salt | Potassium Sodium Tartrate | KNaC 4 H 4 O 6 |
| Seignette salt | Potassium Sodium Tartrate | KNaC 4 H 4 O 6 |
| Tartar | Potassium hydrogen tartrate | KHC 4 H 4 O 6 |
Aromatic hydrocarbons
Carbohydrates and derivatives
| Common name | Chemical name | chemical formula |
|---|---|---|
| Dextrose | glucose | C 6 H 12 O 6 |
| glucose | ||
| Fructose | Fructose | C 6 H 12 O 6 |
| Levulosis | ||
| Malt sugar | Maltose | C 12 H 22 O 11 |
| Lactose | Lactose | C 12 H 22 O 11 |
| sugar | Sucrose | C 12 H 22 O 11 |
| Dinitrocellulose | Cellulose dinitrate | (C 6 H 8 O 9 N 2 ) n |
| Collodion wool | ||
| Nitrocellulose | Cellulose trinitrate | (C 6 H 7 O 11 N 3 ) n |
| Gun cotton | ||
| Acetyl cellulose | Cellulose diacetate | (C 10 H 14 O 7 ) n |
Organometallic compounds
| Common name | Chemical name | chemical formula |
|---|---|---|
| Grignard connection | Alkyl or aryl magnesium halide | R – Mg – X (R = alkyl / aryl , X = halogen ) |
| Metallocenes ( ferrocene etc.) | Bis (cyclopentadienyl) metal ( bis (cyclopentadienyl) iron etc.) |

|
| Tebbe reagent | μ-Chlorobis (cyclopentadienyl) - (dimethylaluminum) -μ-methylene titanium |
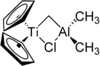
|
literature
- Christian Wiegand: "Origin and interpretation of important organic trivial names. I. Hydrocarbons of the benzene series", in: Angewandte Chemie , 1948 , 60 (4), pp. 109–111 ( doi : 10.1002 / anie.19480600407 ).
- Christian Wiegand: "Origin and interpretation of important organic trivial names. II. Polynuclear isocyclic hydrocarbons", in: Angewandte Chemie , 1948 , 60 (5), pp. 127–129 ( doi : 10.1002 / anie.19480600506 ).
- Christian Wiegand: "Origin and interpretation of important organic trivial names. III. Heterocyclic compounds", in: Angewandte Chemie , 1948 , 60 (7/8), pp. 204-207 ( doi : 10.1002 / anie.19480600709 ).
- M. Binnewies et alii: General and Inorganic Chemistry . 2nd Edition. Spectrum, 2011, ISBN 3-8274-2533-6 , pp. 9-11.