Sodium persulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium persulfate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
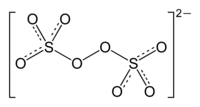
| Molecular formula | Na 2 S 2 O 8 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 238.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.20 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
Decomposition from approx. 180 ° C |
||||||||||||||||||
| solubility |
good in water (545 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sodium persulfate (chemical formula Na 2 S 2 O 8 ) is the sodium salt of peroxodisulfuric acid . Since it tends to disintegrate with the formation of oxygen - especially when it is impure or moist - the salt and its solutions must never be stored in tightly closed containers due to the risk of bursting.
The persulfate ion contains oxygen in the unstable oxidation state −1, so sodium persulfate acts as a very strong oxidizing agent . To distinguish it from potassium peroxomonosulfate, iodine is slowly separated from iodide solutions , manganese (II) solutions are oxidized to manganese dioxide, and in the presence of catalytically active silver ions even to permanganate .
use
Dissolved in water , sodium persulfate is used in circuit board technology to etch the copper layers of circuit boards :
Instead of sodium persulfate, the corresponding ammonium salt can also be used.
Compared to the usual etching with iron (III) chloride solution, sodium persulfate has the advantage of less underetching even at higher temperatures (not above 50 ° C, increased decay), and thus more precise contour definition (hence the trade name Feinätzkristall ).
After use, the solution must be disposed of as hazardous waste due to its copper content, or the copper must be removed from the solution by reducing the amount of steel wool.
Sodium persulfate is also used as a water-soluble initiator for free radical polymerization (e.g. emulsion polymerization ).
It is rarely used in pyrotechnics . By mixing magnesium powder with sodium persulfate, what is known as flash powder is obtained.
Individual evidence
- ↑ Entry on SODIUM PERSULFATE in the CosIng database of the EU Commission, accessed on April 17, 2020.
- ↑ a b c d e f entry to sodium in the GESTIS database of IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Disposal of caustic liquids ( Memento of September 7, 2009 in the Internet Archive )



