Melting point
| material | ° C | K |
|---|---|---|
| Helium (at 26 bar) | −272.2 | 0.955 |
| hydrogen | −259 | 14th |
| deuterium | −254 | 19th |
| Tritium | −253 | 20th |
| neon | −248 | 25th |
| oxygen | −218 | 55 |
| nitrogen | −210 | 63 |
| ozone | −193 | 80 |
| Ethanol (C 2 H 5 OH) | −114 | 159 |
| chlorine | −102 | 171 |
| Motor gasoline | −40 | 233 |
| mercury | −38.36 | 234.795 |
| Glycol dinitrate | −22 | 251 |
| water | 0 | 273.155 |
| Nitroglycerin | 2 | 275.95 |
| benzene | 5.5 | 278.7 |
| candle wax | 55 | 328 |
| naphthalene | 80 | 353 |
| Trinitrotoluene | 80.35 | 353.20 |
| Sulfur ( rhombic ) | 113 | 386 |
| Sulfur ( monoclinic ) | 119 | 392 |
| sugar | 160 | 433 |
| lithium | 180 | 453 |
| tin | 231 | 504 |
| lead | 327.4 | 600.6 |
| zinc | 419.5 | 692.7 |
| aluminum | 660.32 | 933.48 |
| Table salt | 801 | 1074 |
| silver | 960.8 | 1234.0 |
| gold | 1064 | 1337 |
| copper | 1084 | 1357 |
| beryllium | 1287 | 1560 |
| iron | 1536 | 1809 |
| platinum | 1773.5 | 2046.7 |
| boron | 2076 | 2349 |
| tungsten | 3422 | 3695 |
| Hafnium Carbide (HfC) | 3890 | 4163 |
| Tantalum carbide | 3942 | 4215 |
| Tantalum hafnium carbide | 4215 | 4488 |
As a melting temperature (commonly known as melting point (mp.), Engl. Melting point (Mp.)) Is defined as the temperature at which a substance will melt , i.e. from the solid to the liquid aggregate state transitions. The melting temperature depends on the substance, but in contrast to the boiling temperature, it depends only very little on the pressure (melting pressure). Melting temperature and pressure are referred to together as the melting point , which describes the state of a pure substance and is part of the melting curve in the phase diagram of the substance. Some substances cannot melt because they decompose chemically beforehand, and others can only sublime under normal conditions .
For pure chemical elements, the melting point is identical to the freezing point and remains constant during the entire melting process. The melting temperature is generally lowered by impurities or in the case of mixtures ( lowering of the melting point ); in addition, the temperature can rise during the melting process, which means that a melting range is involved. The lowering of the melting point ( cryoscopy ) caused by dissolved substances is one reason why ice can be melted by salt.
In contrast to chemical elements, even with pure chemical compounds there can be deviations between the melting point and the freezing point. If the freezing point temperature is below the melting point temperature, one speaks of a thermal hysteresis. This is the case, for example, with pure water ; Without nucleation nuclei and under a pressure of 1 bar, water freezes at approx. −40 ° C and melts at approx. 0 ° C. For amorphous materials such as B. Glasses and some plastics are referred to as the transition temperature . It is also possible to define a softening temperature .
The melting temperature, along with density , fracture toughness , strength , ductility and hardness , is one of the material properties of a material .
The principal liquid range of 630 ° C to 3900 ° C, ie over 3270 ° C, has the element neptunium . The smallest liquid range from −248.6 ° C to −246.3 ° C has the noble gas neon at 2.3 ° C.
Pressure dependency
The melting point depends on the pressure , but only slightly: To change the melting point by just 1 K , the pressure has to be increased by an average of around 100 bar . It follows that changes in atmospheric pressure - which can cause noticeable changes in boiling point - have virtually no effect on melting point.
For melting, as for other phase transformations, the Clapeyron equation applies , which gives the following temperature change Δ T as a good approximation for melting at different pressures :
T M is the melting point, Δ V is the change in volume during melting, Δ p is the difference between the pressures under consideration, and H M is the enthalpy of fusion . However, since the volume changes Δ V during melting are relatively small, the pressure dependence of the melting point is also relatively small. For example, if the pressure is increased by 100 bar, the melting point of ice changes by −0.76 K. Ice therefore melts more easily under pressure, while the melting point of carbon tetrachloride increases by +3.7 K. The fact that the melting point of ice or, for example, bismuth, decreases when the pressure increases, follows from the fact that its volume is reduced when it melts: Then Δ V and Δ T in the above equation are negative.
Analytics
The determination of the melting point of a substance is also of great importance in qualitative analysis , including identity testing , since many substances can be identified by their melting point. The purity of substances can also be measured qualitatively using the melting point. Impurities result in lower melting points. Liquid substances or those with a low melting point are converted into easily crystallizing derivatives : Alcohols can be identified, for example, by measuring the melting points of their esters of 4-nitrobenzoic acid or 3,5-dinitrobenzoic acid . For this purpose, the substance to be analyzed is converted in the presence of small amounts of sulfuric acid. The melting points of these derivatives are usually sharp.
 |
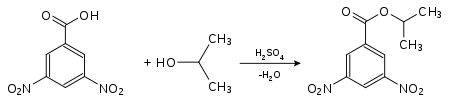
|
| Detection of isopropanol as a derivative of 4-nitrobenzoic acid: 4-nitrobenzoic acid-2-propyl ester (melting point: 100.5 ° C) |
Detection of isopropanol as a derivative of 3,5-dinitrobenzoic acid: 3,5-dinitrobenzoic acid-2-propyl ester (melting point: 123 ° C) |
The derivatives of 3,5-dinitrobenzoic acid generally have higher melting points than those of 4-nitrobenzoic acid. They are preferred when the melting point of 4-nitrobenzoic acid is too low and an exact determination is no longer possible.
There are extensive tables with information on the melting points of organic compounds as important aids for analysts. Melting points of derivatives of individual substance classes are listed in the relevant textbooks on organic analysis.
determination
An approximate measurement is possible with a thermometer by melting the sample and reading off the melting temperature.
Various methods are available for the exact measurement of the melting point:
- Apparatus according to Thiele , in which the sample is melted in a stirred or convection- flowing oil bath
- Apparatus according to DAB , with standard ground joint 29/32, consisting of a flask of approx. 100 ml and an insert tube with a vent hole
- Apparatus according to Dr. CF Linström (often incorrectly spelled Lindström ), here the sample is heated to the melting point in a copper block
- Heating table apparatus according to Kofler (see also Kofler heating bench ), Tottoli
- Dynamic differential calorimetry (DSC)
Usually the measured values are marked with the fact that they have not been corrected . This information relates to the (minor) error that arises from the fact that only the reservoir of the thermometer is immersed in the measuring medium, but not the ascending thread, which therefore has a different temperature and expansion.
In practical laboratory operations today, mostly automatic melting point measuring devices are used, which deliver the result digitally in a short time.
See also
- Molar lowering of the melting point
- Instant melting point
- Thermodiffere
Web links
- Melting points of the chemical elements of the periodic table can be sorted according to various criteria (ordinal number, name, value in ascending or descending order).
Individual evidence
- ↑ Thomas Jüstel: Chemistry Records (pdf file). Accessed June 2020.
- ↑ a b c CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ W. Utermark, W. Schicke: Melting point tables of organic compounds , 2nd edition. Akademie Verlag, Berlin 1963 DNB 455194963 .
- ^ RL Shriner, RC Fuson, DY Curtin, TC Morrill: The Systematic Identification of Organic Compounds , John Wiley & Sons, New York - Chichester - Brisbane - Toronto 1980, ISBN 0-471-78874-0 .
- ↑ CFLinström: A new melting point determination apparatus made of copper. in: Chem. Fabrik 7, 270 (1934); Note: Carl Friedrich Linström was assistant at the Phys.-Chemisches Institut in Erlangen under G. Scheibe.
- ↑ M. Tottoli: Swiss Patent 320388 doi : 10.1007 / BFb0050861 .



