Sublimation (phase transition)
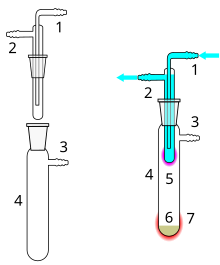
1 Cooling water inlet
2 Cooling water outlet
3 Vacuum connection
4 Sublimation chamber
5 Sublimated material
6 Unsublimated material
7 External heat supply
As sublimation , rarely sublimation (of lat. Sublimis , high in the air located ',' superior '), is the process of immediate transition of a substance from the solid to the gaseous state of aggregation without liquefy before. It is a purely physical process in which the substance remains chemically unchanged.
Under the pressure and temperature conditions under which sublimation occurs, there is no liquid aggregate state, as can be seen in the phase diagram on the right. These conditions are also referred to as sublimation pressure and sublimation temperature, or taken together as the sublimation point . This in turn is part of the sublimation curve of the phase diagram, which is given in the adjacent example by the phase boundary line between solid and gas below the triple point .
The phase change in the opposite direction to sublimation is referred to in technical terms as resublimation or as deposition or desublimation. The resublimation point for pure substances is identical to the sublimation point. In the case of mixtures , it must be noted that both can differ and therefore the direction of the phase transition also plays a role in this case .
If there is a sublimation temperature at normal pressure, this is called the normal sublimation temperature and the substance is tabulated with its value without additionally specifying the sublimation pressure.
Every substance absorbs what is known as the heat of sublimation during its sublimation , which is equal to the sum of the heat of fusion and heat of vaporization .
Examples
- Sublimation in the real sense
- Boron , carbon and arsenic , but also organic compounds such as camphor, are converted directly into gaseous form when heated under normal pressure .
- Dry ice , at -78.5 ° C frozen carbon dioxide is sublimated when heat and directly enters into the gaseous state of aggregation above. Under normal pressure conditions, no liquid such as B. when heating water ice : this is where the term dry ice is derived .
- Sublimation above the triple point
- If the air is sufficiently cold and dry at atmospheric pressure, water also passes directly from its solid state of aggregation into gaseous form ( water vapor ). The reason for this is that the water vapor pressure is a partial pressure of the air and is therefore lower than the atmospheric pressure of the air. This is why this phase transition takes place in the phase diagram for water in the sublimation area below the triple point, even if it appears to occur at atmospheric pressure. Thanks to this effect, damp laundry dries faster in the outside air under the corresponding conditions in frosty conditions than in interior rooms, where the warmer air is already more or less saturated with water vapor.
- Iodine sublimes at normal pressure when heated, whereby this is particularly effective as a violet gas and is therefore often used as a show experiment. In fact, the pressure at the triple point (386.65 K, 12.1 kPa) is far below the ambient pressure (101.315 kPa). However, as long as the temperature is kept below the melting point (113.70 ° C), iodine actually changes from the solid to the gaseous state. In addition, iodine first melts into a deep purple to black liquid.
Confusion with dissociation
Sublimation can be confused with dissociation . For example, ammonium chloride breaks down into ammonia and hydrogen chloride when heated .
application
When processing materials by means of laser beam sublimation cutting, the sublimation occurs with the high pulse power of the laser. The material does not melt first, but changes directly from the solid to the gaseous state. The result is a very clean cut with no slag or frayed edges.
Further applications are the freeze-drying of food, which is very gentle compared to other drying methods, as well as sublimation printing .
Sublimation is also an alternative to recrystallization when purifying products in synthetic chemistry. Compared to crystallization , sublimation offers several advantages: The products are often very clean and even the smallest amounts can be conveniently sublimated in a sublimation apparatus. The authors present an example of the application of sublimation of small amounts in the radiochemical field for radiochemical purification of a nuclide in the context of the half-life determination of 79 Se . Sublimation is primarily a laboratory process for cleaning fabrics, e.g. B. for ferrocene and pyrogallol . A disadvantage compared to crystallization from the melt is the comparatively difficult scaling up during sublimation. In the chemical industry, the technical implementation of sublimation as a separation method is therefore of secondary importance. Sublimation is used industrially as a refining process for naphthalene , phthalic anhydride , camphor , anthraquinone , salicylic acid , benzoic acid , uranium hexafluoride and many metals.
Transport reactions
Chemical transport reactions are used to purify substances (mostly metals) by means of sublimation. Here, however, the substance to be cleaned is first converted into a secondary substance in chemical reactions, which z. B. has a lower sublimation pressure. Examples of transport reactions are the Mond process or the Van Arkel de Boer process .
Individual evidence
- ↑ Entry on sublimation . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S06069 .
- ↑ Michael Wächter: Chemielabor - Introduction to Laboratory Practice , Wiley-VCH Verlag, Weinheim, 1st edition, 2011, p. 76, ISBN 978-3-527-32996-0 .
- ↑ jom: JUST ASK ! : Evaporated ice cold . In: badische-zeitung.de, Ratgeber, Bildung & Wissen, December 10, 2011 (December 16, 2011).
- ↑ NileRed: The Iodine Myth. Retrieved September 13, 2017 . The melting and the deep purple color of the liquid iodine are clearly visible.
- ↑ Heinz GO Becker et al .: Organikum , Johann Ambrosius Barth Verlag Leipzig, Berlin, Heidelberg, pp. 53–54, ISBN 3-335-00343-8 .
- ↑ Physikalisch-Technische Bundesanstalt: The half-life of 79 Se , News 2010.
- ↑ Jörg, G., Bühnemann, R., Hollas, S., Kivel, N., Kossert, K., Van Winckel, S., Lierse v. Gostomski, Ch. Applied Radiation and Isotopes 68 (2010), 2339-2351.
- ^ TJ Kealy, PL Pauson: A New Type of Organo-Iron Compound . Nature 1951, 168, 1039. doi : 10.1038 / 1681039b0 .
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 5: Pl-S. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1987, ISBN 3-440-04515-3 .

