ammonia
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | ammonia | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | NH 3 | |||||||||||||||
| Brief description |
colorless, pungent smelling gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 17.03 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.7714 kg m −3 (0 ° C, 1013 mbar) |
|||||||||||||||
| Melting point |
−77.7 ° C |
|||||||||||||||
| boiling point |
−33 ° C |
|||||||||||||||
| Vapor pressure |
8573 h Pa (20 ° C) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Dipole moment | ||||||||||||||||
| Refractive index |
1.325 (16.85 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 20 ml m −3 or 14 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−45.9 kJ mol −1 (g) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ammonia [ amoˈni̯ak ], also: [ ˈamoni̯ak ], Austrian: [ aˈmoːniak ] is a chemical compound of nitrogen and hydrogen with the empirical formula NH 3 . It is a colorless, water-soluble and poisonous gas with a strong pungent smell that causes tears and is suffocating. Ammonia is an amphoteric substance: under aqueous conditions it acts as a base . It forms several series of salts : the cationic ammonium salts as well as the anionic amides , imides and nitrides in which one (amide), two (imides) or all (nitrides) protons (hydrogen ions) are replaced by metal ions.
Ammonia is one of the most widely produced chemicals and basic material for the production of all other nitrogen compounds. Most of the ammonia is processed into fertilizers , especially urea and ammonium salts. So far (2020) production has been carried out almost exclusively using the Haber-Bosch process from the elements hydrogen and nitrogen.
In biological terms, ammonia has an important function as an intermediate product in the formation and breakdown of amino acids . Due to the toxicity of larger amounts of ammonia, it is converted into non-toxic urea or, for example in birds, into uric acid for excretion in the body .
history
Naturally occurring ammonium compounds have been known for a long time. Thus, ammonium chloride (ammonium chloride) in antiquity in Egypt by heating camel dung won. When heated, ammonia is formed, which reacts with hydrogen chloride to form ammonium chloride as white smoke. Both salmia and ammonia are derived from the Latin sal ammoniacum , which in turn goes back to the ancient name of the oasis Siwa (oasis of ammon or Amun ). There were large deposits of salt near the oasis, but it was probably sodium chloride and not naturally occurring ammonium chloride.
Gaseous ammonia was first mentioned in 1716 by Johannes Kunckel , who observed fermentation processes . The gas was first isolated in 1774 by Joseph Priestley . Further research was carried out by Carl Wilhelm Scheele and Claude-Louis Berthollet , who recognized the composition of ammonia from nitrogen and hydrogen, and William Henry , who determined the exact ratio of the two elements of 1: 3 and thus the chemical formula NH 3 . Georg Friedrich Hildebrandt carried out the first, but unsuccessful, attempts to synthesize ammonia from the elements around 1795 by letting nitrogen and hydrogen stand over water in various proportions.
Ammonia was needed in larger quantities from 1840 after Justus von Liebig had developed nitrogen fertilization to improve yields in agriculture. Initially, ammonia was obtained as a by-product of the distillation of coal, but after a short time this was no longer sufficient to meet the demand for fertilizers. A first technical process to obtain larger amounts of ammonia was the Frank Caro process in 1898 , in which calcium carbide and nitrogen were converted into calcium cyanamide and this was then converted with water into ammonia.
From around 1900 Fritz Haber , but also Walther Nernst , began researching the direct reaction of nitrogen and hydrogen to form ammonia. They soon realized that this reaction only takes place to a very small extent under normal conditions and that high temperatures, high pressure and a suitable catalyst are necessary for high yields . In 1909 Haber succeeded for the first time in producing ammonia on a laboratory scale by direct synthesis with the help of an osmium catalyst . Thereupon, with the help of Carl Bosch , he tried this process, the later Haber-Bosch process , also to use on an industrial scale. This was achieved after overcoming the technical problems caused by working under high pressure in 1910 in the test operation. In 1913, the first commercial ammonia synthesis factory went into operation at BASF in Ludwigshafen . An iron mixed catalyst developed by Alwin Mittasch was used instead of the expensive osmium. This process was applied on a large scale after a short time and is still used today for ammonia production. In 1918 Fritz Haber received the Nobel Prize in Chemistry for the development of ammonia synthesis, and in 1931, together with Friedrich Bergius , Carl Bosch also received the development of high pressure processes in chemistry.
For a long time, however, nothing precise was known about the exact course of the reaction on the catalyst. Since these are surface reactions, they could only be examined after the development of suitable techniques such as the ultra-high vacuum or the scanning tunneling microscope . The individual partial reactions in ammonia synthesis were discovered by Gerhard Ertl , who also received the 2007 Nobel Prize in Chemistry for this.
The reaction of ammonia to nitric acid was first investigated by Frédéric Kuhlmann from 1825 . A technically applicable process for the synthesis of nitric acid from ammonia was developed by Wilhelm Ostwald with today's Ostwald process at the beginning of the 20th century . After the development of the Haber-Bosch process, this also became technically important and soon largely replaced the previous production process using expensive Chile nitrate .
Occurrence
Since ammonia reacts easily with acidic compounds, free ammonia gas occurs only in small quantities on earth. It arises z. B. in the decomposition of dead plants and animal excrement. In so-called humification , nitrogenous components of the biomass are broken down by microorganisms in such a way that, among other things, ammonia is produced. This gets into the air as a gas, but reacts there with acids such as sulfuric or nitric acid and forms the corresponding salts. These can also be transported over long distances and easily get into the ground. Almost all of the ammonia emissions come from livestock farming . To a lesser extent, volcanic gases can also contribute to environmental pollution , as can road traffic .
Ammonium salts, on the other hand, are widespread on earth. The most common ammonium salt is salmia ( ammonium chloride ), but also diammonium hydrogen phosphate ( phosphammite ), ammonium sulfate ( mascagnine ) and a number of complex ammonium salts with other cations are known from nature. These are mainly found in the vicinity of volcanoes or burning coal seams , in which organic substances are broken down into ammonia, among other things. For example, ammonia is mainly found as a sublimation product around fumaroles , where the hydrogen chloride and ammonia gases contained in the hot steam are precipitated as ammonium chloride.
Many rocks and sediments, especially muscovite , biotite and feldspar minerals, also contain ammonium. In contrast, quartz rocks contain only small amounts of ammonium. In addition to the origin of the ammonium, the escape of ammonia during metamorphosis also plays a role in the distribution.
Ammonia is also found in space . In 1968 it was the first molecule to be found in interstellar space through its microwave spectrum . Ammonia is also found on the gas planets of the solar system.
Extraction and presentation
Ammonia is a basic chemical and is produced on a large scale. In 2017 150 million tons were produced worldwide. The main producers are the People's Republic of China , India , Russia and the United States . Large quantities of fossil fuels are currently required for ammonia production. The share of ammonia production in the global consumption of fossil fuels is around 1.4 to 3%. In 2007, the production of one tonne of ammonia in Germany required 11.7 MJ of energy and caused around 1.4 t of CO2 emissions.
Over 90% of the ammonia produced is produced in direct synthesis using the Haber-Bosch process . The gases nitrogen and hydrogen react with each other in a heterogeneous catalytic reaction in large reactors .
- Reaction equation for direct synthesis from nitrogen and hydrogen.

The reaction is exothermic , but has a high activation energy , which is why a catalyst is needed to achieve a significant conversion rate.
Before the actual reaction, the starting materials must first be obtained. While nitrogen is available in large quantities as a constituent of air and is obtained by liquefying the air , hydrogen must first be produced from suitable sources. The most important process currently (2020) is steam reforming , in which primarily natural gas , but also coal and naphtha , are converted into hydrogen and carbon dioxide in two steps with water and oxygen . After the carbon dioxide has been separated off, the hydrogen is mixed with nitrogen in the correct ratio and, depending on the process , compressed to 80-400 bar , typically 150-250 bar. In the future, it is to be expected that hydrogen will be generated more and more electrolytically from water using electricity generated from renewable sources.
The gas mixture is fed into the reaction circuit. There it is first cooled to remove traces of water and then heated to 400–500 ° C using heat exchangers . The hot gas mixture can then react in the actual reactor to form ammonia on iron catalysts that are mixed with various promoters such as aluminum oxide or calcium oxide . For economic reasons, the gases are only exposed to the catalysts for a short time in industrial operation, so that equilibrium cannot be established and the reaction is incomplete. The gas mixture, which now has an ammonia content of around 16.4%, is cooled in several stages so that the ammonia becomes liquid and can be separated off. The remaining mixture of nitrogen, hydrogen and a small amount of ammonia is fed back into the cycle together with fresh gas.
A possible catalyst alternative would be ruthenium , which has a significantly higher catalyst activity and thus enables higher yields at low pressures. Due to the high price of the rare precious metal ruthenium, however, such a catalyst has so far only been used to a limited extent on an industrial scale.
In 2014, an alternative synthesis to the Haber-Bosch process with significantly lower energy consumption was presented. Here serve nanoparticles of iron (III) oxide which a equimolar mixture of potassium hydroxide and sodium hydroxide , can be added as a catalyst. The mixture is heated to 200 ° C. and put under voltage (1.2 V). Water vapor and air are added, whereupon ammonia forms. The efficiency based on the electrical charge used (Faraday efficiency) is 35%.
Several working groups are working on a CO 2 -neutral ammonia production based on electrochemical processes ("electrochemical ammonia synthesis"). The hydrogen generated by the electrolysis of water is supposed to react directly with nitrogen to form ammonia in the presence of certain catalysts and membranes. In the future, the electricity will mainly come from renewable sources.
properties
Physical Properties
Ammonia is a colorless, diamagnetic , pungent smelling gas at room temperature . Below -33 ° C it becomes liquid. The liquid is colorless and highly refractive and has a density of 0.6819 kg / l at the boiling point. The gas can also be easily liquefied by increasing the pressure; at 20 ° C a pressure of 900 kPa is sufficient. The critical temperature is 132.4 ° C, the critical pressure is 113 bar, the critical density is 0.236 g / cm 3 . Ammonia is explosive within the range from 15.4 to 33.6% by volume (108–336 g / m 3 ). Its ignition temperature is 630 ° C.
In the liquid phase, ammonia forms hydrogen bonds , which is the reason for the relatively high boiling point and a high enthalpy of vaporization of 23.35 kJ / mol. To break these bonds during evaporation, a lot of energy is needed, which has to be supplied from the environment. Therefore, liquid ammonia is suitable for cooling. Before the use of halogenated hydrocarbons , ammonia was a widely used refrigerant in refrigerators.
Below −77.7 ° C, ammonia solidifies in the form of colorless crystals. It crystallizes in the cubic crystal system with a lattice parameter a = 508.4 pm (−196 ° C). At −102 ° C the lattice parameter is a = 513.8 pm. The structure can be derived from a face-centered cubic lattice , with six of the twelve neighboring molecules being closer to the central molecule than the remaining six. Each lone pair of electrons is coordinated with three hydrogen atoms.
Density anomaly and (non-isobaric) expansion coefficient of liquid ammonia
At every temperature the liquid gas has a different vapor pressure , according to its vapor pressure function . Therefore, the temperature-related expansion or contraction of the volume does not take place isobarically .
| substance | / in [° C] | / in [g / cm³] | in [K] | mean temperature in [° C] | in [1 / K] | swell |
|---|---|---|---|---|---|---|
| liquid ammonia, boiling (at its own vapor pressure) | −70 / −68 | 0.72527 / 0.72036 | 2 | −69 | +0.003408 | |
| −68 / −66 | 0.72036 / 0.72067 | 2 | −67 | -0.000215 | ||
| −66 / −64 | 0.72067 / 0.71839 | 2 | −65 | +0.001587 | ||
| -64 / -62 | 0.71839 / 0.71608 | 2 | −63 | +0.001613 | ||
| −50 / −48 | 0.70200 / 0.69964 | 2 | −49 | +0.001687 | ||
| −30 / −28 | 0.67764 / 0.67517 | 2 | −29 | +0.001829 | ||
| −28 / −26 | 0.67517 / 0.67263 | 2 | −27 | +0.001888 | ||
| −26 / −24 | 0.67263 / 0.67463 | 2 | −25 | -0.001482 | ||
| −24 / −22 | 0.67463 / 0.68587 | 2 | −23 | -0.008194 | ||
| −22 / −20 | 0.68587 / 0.66503 | 2 | −21 | +0.015668 | ||
| −2 / 0 | 0.64127 / 0.63857 | 2 | −1 | +0.002114 | ||
| −2 / 2 | 0.64127 / 0.63585 | 4th | 0 | +0.002131 | ||
| 0/2 | 0.63857 / 0.63585 | 2 | 1 | 0.002139 | ||
| 18/20 | 0.61320 / 0.61028 | 2 | 19th | +0.002392 | ||
| 18/22 | 0.61320 / 0.60731 | 4th | 20th | +0.002425 | ||
| 20/22 | 0.61028 / 0.60731 | 2 | 21st | +0.002445 | ||
| 24/26 | 0.60438 / 0.60132 | 2 | 25th | +0.002544 | ||
| 48/50 | 0.56628 / 0.56306 | 2 | 49 | +0.002859 |
Note: Density values and expansion coefficients of liquid ammonia show two density anomalies in the temperature range under consideration !
The mean expansion coefficients were calculated from the density values:
The density quotients are indirectly proportional to the volume quotients or the quotients of the specific volumes v ( mass- specific or molar volume )!
Molecular Properties
Ammonia consists of one nitrogen and three hydrogen atoms. These are not arranged in one plane, but in the form of a three-sided pyramid ( trigonal - pyramidal ). The nitrogen atom forms the top, the hydrogen atoms the base of the pyramid. A lone pair of electrons in nitrogen is responsible for this form . If this is taken into account, the structure corresponds to that of a distorted tetrahedron . According to the VSEPR model , the lone pair of electrons results in a deviation from the ideal tetrahedral angle (109.5 °) to a hydrogen-nitrogen-hydrogen angle of 107.3 °. This lies between the bond angles in methane (ideal tetrahedron angle of 109.5 °) and water (greater distortion due to two free electron pairs, angle 104.5 °). The bond length of the nitrogen-hydrogen bond in ammonia is 101.4 pm, which in turn lies between the bond lengths in methane of 108.7 pm and water (95.7 pm). This can be explained by the increasing electronegativity difference between carbon via nitrogen and oxygen and thus a stronger polar bond .
The ammonia molecule is not rigid, the hydrogen atoms can fold over to the other side of the pyramid via a planar transition state. At 24.2 kJ / mol, the energy barrier for pyramidal inversion is so small that no enantiomers can be isolated at room temperature from ammonia and amines NR 3 (R: organic residues ) derived from it. Ammonia molecules have a very precise and constant oscillation frequency of 23.786 GHz , which can be used to measure time. Among other things, the first atomic clock was constructed using the ammonia oscillation frequency.
Chemical properties
Liquid ammonia
Liquid ammonia is a good solvent and has properties similar to water. It dissolves many organic compounds, such as alcohols , phenols , aldehydes and esters and many salts, solvating the ions that form.
The liquid undergoes autoprotolysis in analogy to water with the ion product of only 10 −29 mol 2 / l 2 and a neutral point of 14.5:
Liquid ammonia reacts with elemental alkali metals , as well as elemental calcium , strontium and barium , forming deep blue solutions. Solvated metal ions and solvated electrons are formed . The color is caused by solvated electrons that are present in solution without bonding to any particular atom. These also cause the solutions to have good electrical conductivity .
Such solutions are used to reduce aromatics, see Birch reduction . The solution is stable for a long time, in a redox reaction a metal amide M'NH 2 is slowly formed with the release of elemental hydrogen , in the presence of a catalyst such as iron (II) chloride :
Aqueous solutions
Ammonia is very soluble in water. At 0 ° C, 1176 liters of ammonia dissolve in one liter of water. The solutions are called ammonium hydroxide , ammonia or ammonia water and have a basic reaction.
As a base with a base constant p K b of 4.76, ammonia reacts with water to form hydroxide ions (OH - ):
However, the equilibrium of the reaction is largely on the side of ammonia and water. Ammonia is therefore largely in the form of a molecularly dissolved compound. No amide ions (NH 2 - ) are formed in aqueous solutions , since these would be a very strong base in water with p K b = −9 and ammonia therefore does not react as an acid ( proton donor ). With a strong acid , ammonia converts to ammonium ions (NH 4 + ):
In the classic sense of a neutralization of ammonia, a solution of ammonium salt is formed. Ammonium chloride is formed with hydrochloric acid :
Redox reactions
Ammonia can react with oxygen and burn to nitrogen and water. Ammonia can be ignited in the air, but the energy released is not sufficient for continuous combustion; the flame goes out. In contrast, ammonia burns well in pure oxygen; at higher pressure this reaction can also take place explosively. A corresponding reaction also takes place with strong oxidizing agents such as halogens , hydrogen peroxide or potassium permanganate . According to DIN 51850, ammonia has a calorific value of 17.177 MJ / kg.
In the presence of platinum - or rhodium - catalysts reacts ammonia and oxygen not to nitrogen and water, but nitrogen oxides such as nitric oxide . This reaction is used in the production of nitric acid in the Ostwald process .
With particularly reactive metals such as alkali or alkaline earth metals and in the absence of water formed in a redox reaction amides of the general form M I NH 2 (M I : monovalent metal atom), such as. B. sodium amide .
Compounds of the form M 2 NH in which two of the three hydrogen atoms have been replaced are called imides and if there are no hydrogen atoms, they are called nitrides . They can be obtained by heating amides.
Imide : z. B. Magnesiumimide Nitrides : z. B. Magnesium Nitride
The alkali and alkaline earth salts react with water to form metal hydroxides and ammonia.
Ammine complexes
Ammonia tends to complex formation with many transition metals . Resistant complexes are known especially from Cr 3+ , Co 3+ , Pd 2+ , Pt 4+ , Ni 2+ , Cu 2+ . A pure ammine complex has a cation that carries the charge of the metal and the ammonia molecules are grouped as monodentate ligands around a central metal atom. The ligand binds to the central atom via its free electron pair. The formation reactions of the complexes can be described using the Lewis acid-base concept . The ammine complexes have the general structure
- with M n + as the metal cation with n charges and m ligands.
A well-known ammine complex is the copper tetrammine complex [Cu (NH 3 ) 4 ] 2+ , which has a typical blue color and can be used as evidence of copper . Stable complexes can be in the form of salts , e.g. B. win as sulfates and are called ammine salts or ammoniaates.
In addition to ammonia, ammine complexes can also carry other ligands. In addition to the pure chromium hexammine complex, there are also complexes with the general structure
known. The complexes can therefore also have anions or a molecular (uncharged) structure due to the charge compensation by the ionic ligands. An example of this is cisplatin , [Pt (Cl) 2 (NH 3 ) 2 ], a square-planar platinum (II) complex with two amine ligands and two chloride ions, which is an important cytostatic agent .
In 1893 Alfred Werner first set up a theory for the description of complexes on the ammonia-chlorine complexes of cobalt .
use
Basic material for the production of all other industrially produced nitrogen-containing compounds
With a share of 40% in 1995, urea is the most important compound produced from ammonia, which is primarily used as a fertilizer and for the production of urea resins ; Urea is obtained by reacting ammonia with carbon dioxide .
In addition to urea, other nitrogen fertilizers are also made from ammonia. The most important are the ammonium salts ammonium nitrate , phosphate and sulfate . Overall, the share of fertilizers in total ammonia consumption in 2003 was 83%.
Another important substance made from ammonia is nitric acid , which in turn is the starting material for a large number of other compounds. In the Ostwald process , ammonia reacts with oxygen on platinum meshes and thus forms nitrogen oxides, which react with water to form nitric acid. Compounds made from nitric acid include explosives such as nitroglycerin or TNT . Other substances synthesized from ammonia are amines , amides , cyanides , nitrates and hydrazine .
Role in various organic syntheses
Primary carboxamides can be obtained from ammonia and suitable carboxylic acid derivatives such as carboxylic acid chlorides or esters . In contrast, the direct reaction of carboxylic acid and ammonia to form the corresponding amide only takes place at elevated temperatures when the ammonium salt previously formed decomposes. A technically important reaction is that of adipic acid and ammonia to form adipic acid dinitrile . This is further hydrogenated to hexamethylenediamine and is therefore an intermediate product for the manufacture of nylon .
It is possible to produce aniline through the reaction of phenol and ammonia over an aluminum silicate catalyst. However, this synthesis route requires more energy and gives a lower yield than the synthesis and reduction of nitrobenzene and is therefore only used to a small extent when phenol is available inexpensively.
Special applications
The reaction of ammonia with acids is used in flue gas cleaning . It is able to react with sulfuric and nitric acid and thus removes undesirable, environmentally harmful sulfur and nitrogen oxides from the flue gas .
Due to its large specific enthalpy of vaporization of 1368 kJ / kg, ammonia is also used as a refrigerant . Advantages are low flammability , the non-existent contribution to the greenhouse effect or the destruction of the ozone layer and the application range from −60 to +100 ° C. The toxicity of the compound is disadvantageous.
Ammonia solution is also used in olfactory ampoules. The extreme odor stimulus serves as an anti-dissociative strategy , for example in the context of dialectical behavioral therapy .
Ammonia reacts with the tannic acid found in wood and colors the wood dark brown, depending on the concentration of the tannic acid. For example, oak wood is transformed with ammonia or salmia into the dark brown smoked oak . Ammonia is also used for coloring in the production of blueprints ( diazotypes ).
There are also experimental fuel cell systems in which the required hydrogen is obtained at the time it is needed by splitting the ammonia using chemical processes. Another approach that has been researched is to use ammonia as an easily manageable chemical energy storage device, for example to store the excess electricity from fluctuating renewable energies (wind and solar energy) in the long term and on an industrial scale, to close temporal gaps in the supply of these energies and to use renewable energies for to use vehicle propulsion, for example in ship engines. Due to the efficiency of approx. 55-60% in converting electrical energy into chemical energy of ammonia and back in large diesel or combined cycle power plants (together approx. 2/3 energy losses), however, this is significantly less energetic than the direct use of renewable energies for the coverage of the electricity demand.
Biological importance
Only a few microorganisms are able to extract ammonia directly from the nitrogen in the air in what is known as nitrogen fixation . Examples are cyanobacteria or proteobacteria such as Azotobacter . From this ammonia, which is obtained by the enzyme nitrogenase , the bacteria synthesize amino acids that are required by all living things. Most legumes , such as beans , clover and lupins, have also entered into symbioses with certain types of bacteria for a better supply of amino acids .
Ammonia, which is present as ammonium under biochemical conditions, plays an important role in the metabolism during the build-up and breakdown of amino acids. Glutamate is produced from ammonium and α-ketoglutarate through reductive amination , from which further amino acids can in turn be synthesized through transamination . While microorganisms and plants synthesize all amino acids in this way, in humans and animals this is limited to the non-essential amino acids.
The breakdown of amino acids also takes place initially via transamination to glutamate , which is split back into α-ketoglutarate and ammonia by the enzyme glutamate dehydrogenase . Since larger amounts of ammonia have a toxic effect and cannot be used completely for the construction of new amino acids, there must be a possibility of degradation. The way to remove the excess ammonia from the body is decided depending on the species and habitat. Water-dwelling organisms can release ammonium directly into the surrounding water and do not need a non-toxic intermediate storage. Organisms that live in rural areas, on the other hand, have to convert the ammonia into non-toxic intermediate products before excretion. There are essentially two substances that are used. Insects , reptiles and birds use the water-insoluble uric acid , which is excreted as a solid ( uricotelia ). This is beneficial in arid areas and in saving weight in birds. Mammals , on the other hand, are able to convert ammonium into non-toxic and water-soluble urea in the liver via the urea cycle ( ureotelia ). This can then be excreted in the urine.
Urea can be broken down into ammonia and carbon dioxide by the enzyme urease , which is found in some plants such as the soybean or sword bean , in certain bacteria and invertebrates. These bacteria are found in the rumen of ruminants , among other things, and cause the manure and manure of these animals to contain ammonia. This is also the largest anthropogenic source of ammonia in the environment.
Ammonia is of particular importance in the ecology of water. Depending on the pH value , the ratio of the dissolved ammonium ions and the undissolved ammonia in the water shifts, with the concentration of ammonia increasing with increasing pH values, while that of the ammonium ions decreases accordingly. At values up to approx. PH 8, ammonium ions are present almost exclusively, at values above a pH of 10.5, almost exclusively ammonia is present. An increase in the pH value can mainly occur through a strong increase in photosynthetic activity , for example in the case of algal blooms , in weakly buffered and wastewater-polluted waters. Since ammonia is toxic to most aquatic organisms, sudden fish deaths can occur if the critical pH value is exceeded .
toxicology
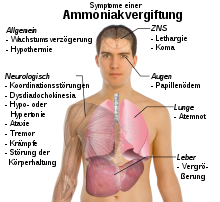
Due to the unpleasant odor, which is noticeable even at low concentrations, there is a warning so that cases of poisoning with ammonia are rare. Gaseous ammonia can mainly be absorbed through the lungs . It has a very corrosive effect on the mucous membranes due to its reaction with moisture . The eyes are also severely damaged by the effects of ammonia. Ppm by inhalation of high concentrations from about 1700 danger to life caused by damage to the respiratory tract (larynx edema , laryngospasm , pulmonary edema , pneumonitis ), and respiratory arrest . When substantial amounts of ammonia pass into the blood, the blood level of NH 4 + rises to over 35 µmol / l, which can cause central nervous symptoms such as tremor of the hands, speech and vision disorders and confusion up to coma and death. The pathophysiological mechanisms are not yet fully understood, ammonia appears to damage the astrocytes in the brain in particular . Acute ammonia poisoning can occur not only through inhalation but also as a result of liver failure (→ hepatic encephalopathy ) or with enzyme defects, since then the N-compounds occurring in the metabolism cannot be converted to urea and excreted ("endogenous ammonia poisoning"). One possible explanation for the nerve-toxic effects of ammonia is the similarity of ammonium to potassium . The exchange of potassium by ammonium leads to disturbances in the activity of the NMDA receptor and, as a result, to an increased calcium influx into the nerve cells , which causes their cell death . The cell poison ammonia mainly affects nerve and muscle cells. Almost all biological membranes are permeable to ammonia due to the small size of the molecule and its lipid solubility. The cytotoxicity is also based on the disruption of the citric acid cycle by aminating the important metabolite α-ketoglutaric acid to glutamic acid, as well as on the disruption of the pH value of the cells. The encephalotoxic effect is also associated with an increased level of glutamine in the brain and the formation of reactive oxygen species .
Chronic effects from prolonged exposure to ammonia are also present. Damage to the airways can lead to bronchial asthma , coughing or shortness of breath . Aqueous ammonia solutions can also be absorbed through the skin and stomach and burn them. Ammonia comes into the air through fertilization and factory farming. There it is converted into ammonium sulfate and nitrate, which significantly contributes to the creation of fine dust particles . In addition, ammonia, together with nitrogen oxides, promotes the formation of ground-level ozone, which is harmful to health. It is estimated that agriculture was the cause of around 45% of all deaths from air pollution in Germany in 2010 . Ammonia is the only air pollutant that has not seen a significant reduction since 1990. A 50% reduction in ammonia emissions could therefore prevent around 250,000 deaths from air pollution worldwide, and even 800,000 deaths if these emissions were completely eliminated.
In domestic cattle, acute ammonia poisoning occurs mainly when feeding non-protein nitrogen compounds (NPN). If the urea content in the feed is more than 1.5%, symptoms of central nervous poisoning occur because the urea can no longer be completely processed by the rumen flora for protein synthesis. Chronic exposure to ammonia in stables in livestock and laboratory animals, especially in forms of housing without straw and higher temperatures with inadequate ventilation, leads to damage to the respiratory tract and thus to an increased incidence of respiratory infections , to reduced feed consumption and performance losses.
Fish and other aquatic organisms are particularly affected by the risk of ammonia poisoning because of the good water solubility of ammonia . While many fish species can only tolerate low concentrations of ammonia, some species have developed special strategies to tolerate higher concentrations. This includes converting the ammonia to less toxic compounds like urea, or even pumps to actively remove ammonia from the body, which has been observed in mudskippers . Ammonia poisoning occurs in pond management and aquaristics. Causes can be the contamination of the water with liquid manure or fertilizers as well as the increase in the pH value with a shift in the dissociation equilibrium towards ammonia. Affected fish show increased blood abundance ( hyperemia ) and bleeding in the gills and internal organs as well as increased mucus production by the skin. At higher concentrations it can lead to the death of parts of the fins, skin areas or gills, to central nervous symptoms or to death.
proof
There are several ways to detect ammonia. Simple evidence, which is often not clear, is the typical smell , the discoloration of indicators due to the basic ammonia or the typical white smoke of ammonium chloride , which is produced when reacting with hydrochloric acid. The reaction of ammonia solutions with copper salt solutions is also characteristic, which produces the dark blue copper tetrammine complex [Cu (NH 3 ) 4 ] 2+ .
A precise reaction for determining ammonia - which is often not used in trace analysis due to the interference with hydrogen sulphide - is the Neßler reaction , in which potassium tetraiodomercurate (II) reacts with ammonia to form a typical brown precipitate of (Hg 2 N) I. Another disadvantage is the use of the toxic mercury .
- Ammonia, potassium tetraiodomercurate (II) and sodium hydroxide solution react to form the iodide salt of Millon's base , which flocculates in aqueous solution, potassium iodide , sodium iodide and water.
Instead, the Berthelot reaction is used, in which ammonia and hypochlorite form chloramines . These are able to react with phenols to form indophenols , which can be recognized by their deep blue color. Kjeldahl's nitrogen determination can also be used for small quantities . Quantitative determinations are also possible with this method.
Measuring ammonia in the air
Optical detection of ammonia in the air is problematic due to its physical properties. Wet chemical processes are used almost exclusively to prevent the simultaneous collection of ammonium in fine dust .
Pollution of the outside air with ammonia can be measured quantitatively with coated diffusion separators , so-called denuders . An acid (e.g. oxalic acid ) is used as the sorbent coating and is analyzed after sampling has been completed.
Alternatively, passive collectors can be used. In contrast to the actively collecting denuders, these devices do not use targeted flow guidance. The ammonia to be detected reaches the sorbent exclusively by diffusion.
Further processes are the indophenol process and the Neßler process. In the indophenol process, the air is passed through a wash bottle filled with dilute sulfuric acid and bound as ammonium sulfate. After conversion to indophenol , its concentration is determined photometrically. In the Nessler process , the ammonium sulphate obtained is reacted with Neßler's reagent and the color intensity of the colloid obtained is determined photometrically. Both methods have in common that they are not selective towards ammonia.
literature
- Max Appl: Ammonia. In: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim 2006 ( doi : 10.1002 / 14356007.a02_143.pub3 ).
- Entry to ammonia. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- Robert Schlögl: Catalytic ammonia synthesis - a “neverending story”? In: Angewandte Chemie . 115 (18), 2003, pp. 2050-2055. doi: 10.1002 / anie.200301553
Web links
Individual evidence
- ^ R. Panico, WH Powell, J.-C. Richer (Ed.): A Guide to IUPAC Nomenclature of Organic Compounds . IUPAC Commission on the Nomenclature of Organic Chemistry. Blackwell Scientific Publications, Oxford 1993, ISBN 0-632-03488-2 , pp. 37 .
- ↑ Entry on AMMONIA in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d e f g h i j k l Entry on ammonia in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ HR Christen, G. Meyer: Fundamentals of general and inorganic chemistry . Diesterweg, 1997, ISBN 3-7935-5493-7 .
- ^ Frederick G. Bordwell, George E. Drucker, Herbert E. Fried: Acidities of Carbon and Nitrogen Acids: The Aromaticity of the Cyclopentadienyl Anion. In: J. Org. Chem. 46, 1981, pp. 632-635 ( doi: 10.1021 / jo00316a032 ).
- ↑ a b c d Entry on ammonia. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-51.
- ↑ PG Sennikov, VE Shkrunin, DA Raldugin, KG Tokhadze: Weak Hydrogen Bonding in Ethanol and Water Solutions of Liquid Volatile Inorganic Hydrides of Group IV-VI Elements (SiH 4 , GeH 4 , PH 3 , AsH 3 , H 2 S, and H 2 Se). 1. IR Spectroscopy of H Bonding in Ethanol Solutions in Hydrides . In: The Journal of Physical Chemistry . tape 100 , no. January 16 , 1996, p. 6415-6420 , doi : 10.1021 / jp953245k .
- ↑ Entry on ammonia, anhydrous in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 2, 2015.
- ^ Archives for industrial pathology and industrial hygiene. Vol. 13, 1955, p. 528.
- ^ American Journal of Emergency Medicine. Vol. 3, 1985, p. 320.
- ↑ Tabulae Biologicae. Vol. 3, 1933, p. 231.
- ^ Federation Proceedings. In: Federation of American Societies for Experimental Biology. Vol. 41, 1982, p. 1568.
- ^ WB Deichmann: Toxicology of Drugs and Chemicals. Academic Press, Inc., New York 1969, p. 607.
- ↑ CRC Handbook, pp. 5–13 ( Memento from April 26, 2015 in the Internet Archive ).
- ↑ Entry on ammonium chloride. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ^ Julius Ruska : Sal ammoniacus, nušādir and Salmiak. In: Meeting reports of the Heidelberg Academy of Sciences: philosophical-historical class. 14, 1923, No. 5, pp. 3-23.
- ^ Leopold Gmelin: Handbook of theoretical chemistry. Volume 1, Franz Varrentramp, Frankfurt 1827, p. 421 ( excerpt in the Google book search).
- ↑ Alwin Mittasch : Comments on catalysis. In: Reports of the German Chemical Society. 59, 1926, pp. 13-36, doi: 10.1002 / cber.19260590103 .
- ↑ a b c d e f g Max Appl: Ammonia. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2006. ( doi : 10.1002 / 14356007.a02_143.pub2 ).
- ^ Bernhard Timm: 50 years of ammonia synthesis. In: Chemical Engineer Technology - CIT. 35, No. 12, 1963, pp. 817-880 ( doi: 10.1002 / cite.330351202 ).
- ↑ Gerhard Ertl: Reactions on surfaces: from atomic to complex (Nobel lecture). In: Angewandte Chemie . 120, 2008, pp. 3578-3590 ( doi: 10.1002 / anie.200800480 ).
- ^ Frédéric Kuhlmann: Treatise on the formation of saltpeter. New generation of nitric acid a. Ammonia. In: Annals of Pharmacy. 29, No. 3, 1839, pp. 272-291 ( doi: 10.1002 / jlac.18390290305 ).
- ^ Lothar Dunsch: The portrait: Wilhelm Ostwald (1853-1932). In: Chemistry in Our Time . 16, No. 6, 1982, pp. 186-196 ( doi: 10.1002 / ciuz.19820160604 ).
- ↑ Relevant air pollution ( Memento from January 30, 2011 in the Internet Archive ): Ammonia . State Institute for Environment, Measurements and Nature Conservation Baden-Württemberg, as of April 2008.
- ↑ ammonia emissions. In: Umweltbundesamt.de . July 30, 2018, accessed January 13, 2019 .
- ↑ Air pollution control in agriculture. In: bafu.admin.ch . July 20, 2018, accessed January 13, 2019 .
- ↑ a b Frank Wlotzka: Investigations on the geochemistry of nitrogen. In: Geochimica et Cosmochimica Acta. 24, 1961, pp. 106-154 ( doi: 10.1016 / 0016-7037 (61) 90010-2 ).
- ^ Salmiak. In: Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols, John W. Anthony: Handbook of Mineralogy. Volume I, 1990, p. 101 (PDF)
- ^ Paul PT Ho, Charles H. Townes: Interstellar Ammonia. In: Annual review of astronomy and astrophysics. 21, 1983, pp. 239-270 ( doi: 10.1146 / annurev.aa.21.090183.001323 ).
- ^ Phil Davies, Kirk Munsell: NASA's Solar Exploration: Multimedia: Gallery: Gas Giant Interiors ( Memento of February 20, 2006 in the Internet Archive ). NASA : accessed August 8, 2009.
- ↑ Nitrogen (fixed) - Ammonia (PDF; 23 kB). In: United States Geological Survey Mineral Commodity Summaries , January 2018.
- ↑ Clean hydrogen from the electric furnace . In: faz.net, May 30, 2019.
- ↑ Fleiter, Tobias; Schlomann, Barbara; Eichhammer, Wolfgang: Energy consumption and CO2 emissions of industrial process technologies - savings potentials, obstacles and instruments in: ISI series "Innovationspotentiale". Stuttgart: Fraunhofer Institute for System Technology and Innovation Research (Fraunhofer ISI), 2013, quoted from "Andrej Guminski, Elsa Rouyrre, Manuel Wiener: CO2 reduction in ammonia production", accessed June 20, 2020
- ↑ "Chemgapedia - Heterogeneous Catalysis" accessed June 20, 2020
- ↑ S. Licht et al.: Ammonia synthesis. Ammonia synthesis by N₂ and steam electrolysis in molten hydroxide suspensions of nanoscale Fe₂O₃. In: Science. 345 (6197), 2014, pp. 637-640. PMID 25104378 ; doi: 10.1126 / science.1254234
- ↑ Kurt Kugler, Alexander Mitos a. a .: Ammonia synthesis 2.0 - electrochemistry versus Haber Bosch. RWTH Topics Energy, Chemical & Process Engineering, Edition 1/2015, pp. 52–55.
- ↑ Rong Lan, John TS Irvine, Shanwen Tao: Synthesis of ammonia directly from air and water at ambient temperature and pressure. In: Scientific Reports. 3, 2013, doi: 10.1038 / srep01145 .
- ^ Developments in Electrochemical Ammonia Synthesis. In: nh3fuelassociation.org. September 27, 2016, accessed July 4, 2017 .
- ↑ a b c d e A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 665.
- ↑ I. Olovsson, DH Templeton: X-ray study of solid ammonia. In: Acta Crystallographica. 12, 1959, pp. 832-836 ( doi: 10.1107 / S0365110X59002420 ).
- ↑ W. Fratzscher, H.-P. Picht: Material data and characteristics of process engineering. Verlag für Grundstoffindindustrie, Leipzig, GDR 1979 / BRD 1993, pp. 144–146, thermodynamic data of ammonia, density values calculated from specific volumes v '
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 529.
- ↑ Christoph Kölmel, Christian Oehsenfeld, Reinhart Ahlrichs: An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines. In: Theor. Chim. Acta. 82, 1991, pp. 271-284 ( doi: 10.1007 / BF01113258 ).
- ^ Paul A Tipler, Ralph A Llewellyn: Modern Physics. 1st edition. Oldenbourg Verlag, 2002, ISBN 3-486-25564-9 , p. 328 ( excerpt in the Google book search).
- ↑ Michael A. Lombardi, Thomas P. Heavner, Steven R. Jefferts: NIST Primary Frequency Standards and the Realization of the SI Second. In: NCSL International measure. 2, No. 4, 2007, pp. 74–89 (PDF)
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit (Ed.): Fachlexikon ABC Chemie. Harri Deutsch, Frankfurt am Main 1987.
- ↑ James E. Huheey: Inorganic Chemistry: Principles of Structure and Reactivity. de Gruyter, Berlin 1988, ISBN 3-11-008163-6 , p. 309 ff.
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexikon der Chemie. Spectrum Academic Publishing House, Heidelberg 2001.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 .
- ^ A b Arnold Willmes: Pocket book chemical substances. Harri Deutsch, Frankfurt am Main 2007.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1315.
- ↑ Entry on ammine salts. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Entry on Amide. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Reinhard Brückner: reaction mechanisms. 3. Edition. Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 329.
- ↑ Thomas Kahl, Kai-Wilfried Schröder, FR Lawrence, WJ Marshall, Hartmut Höke, Rudolf Jäckh: Aniline. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005 ( doi : 10.1002 / 14356007.a02_303 ).
- ↑ a b Heinz Herwig: Heat transfer AZ: Systematic and detailed explanations of important parameters and concepts. Springer, 2000, ISBN 3-540-66852-7 , p. 109.
- ^ Frank Schneider: Specialist knowledge of psychiatry and psychotherapy. Springer DE, 2012, ISBN 978-3-642-17192-5 , p. 357.
- ↑ PhysOrg: Hydrogen breakthrough could be a game-changer for the future of car fuels , accessed July 2, 2014.
- ^ Siemens UK , accessed July 5, 2018.
- ↑ Entry on nitrogen fixation. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ amino acids. In: Lexicon of Biology. Science-online , Spektrum Verlag; Accessed August 7, 2009.
- ↑ Entry on nitrogen excretion. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Entry on urea cycle. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Entry on urease. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ^ Winfried Lampert , Ulrich Sommer : Limnoökologie. Georg Thieme Verlag, Stuttgart 1993, ISBN 3-13-786401-1 , p. 77.
- ^ Georg Löffler: Biochemistry and Pathobiochemistry. 8th edition. Springer, 2006, p. 454.
- ↑ a b D. J. Randall, TKN Tsui: Ammonia toxicity in fish. In: Marine Pollution Bulletin. 45, 2002, pp. 17-23 ( doi: 10.1016 / S0025-326X (02) 00227-8 . PMID 12398363 ).
- ↑ G. Halwachs-Baumann: laboratory medicine: clinic - practice - case studies. Springer, 2006, ISBN 3-211-25291-6 , p. 96.
- ^ GF Fuhrmann: Toxicology for Natural Scientists: Introduction to theoretical and special toxicology. Vieweg + Teubner Verlag, 2006, ISBN 3-8351-0024-6 , p. 53, p. 349.
- ↑ Wissenschaft-Online-Lexika: Entry on ammonia in the lexicon of nutrition ; Retrieved August 26, 2009.
- ↑ J. Hallbach: Clinical chemistry for entry. 2nd Edition. Georg Thieme Verlag, 2006, ISBN 3-13-106342-4 , p. 207.
- ↑ JE O'Connor, BF Kimler, M. Costell, and J. Viña: Ammonia Cytotoxicity Involves Mitochondrial Disfunction, Impairment Of Lipid Metabolism And Oxidative Stress. Dpt. of Biochemistry, University of Valencia, Valencia, Spain; Dpt. of Radiation Biology, Kansas University Medical Center, Kansas City, KS; Instituto de Investigaciones Citológicas, Valencia, Spain.
- ^ Entry on aqueous ammonia solution in the GESTIS substance database of the IFA , accessed on August 9, 2009 (JavaScript required)
- ↑ Leibniz Institute for Tropospheric Research: Modeling of regional and urban air quality , undated
- ↑ Austrian Academy of Sciences: Fine dust sources, factory farming and wood heating. ( Memento from February 12, 2016 in the web archive archive.today ) March 24, 2014.
- ^ Advisory Council for Environmental Issues: Nitrogen: Strategies for solving an urgent environmental problem - short version. January 2015, long version .
- ↑ www.scinexx.de - The Knowledge Magazine: How big is our nitrogen footprint? January 26, 2016.
- ↑ Johannes Lelieveld et al .: The contribution of outdoor air pollution sources to premature mortality on a global scale . In: Nature . tape 525 , 2015, p. 367-371 , doi : 10.1038 / nature15371 .
- ^ Max Planck Society: More deaths from air pollution. 16th September 2015.
- ↑ Trend in air pollutant emissions. In: Umweltbundesamt.de. Retrieved March 10, 2018 .
- ↑ Andrea Pozzer et al .: Impact of agricultural emission reductions on fine-particulate matter and public health . In: Atmospheric Chemistry and Physics . tape 17 , 2017, p. 12813-12826 , doi : 10.5194 / acp-17-12813-2017 .
- ↑ Gerrit Dirksen and others: Internal medicine and surgery of the cattle. 5th edition. Georg Thieme Verlag, 2006, ISBN 3-8304-4169-X , pp. 1133-1134.
- ↑ Wolfgang Methling, Jürgen Unshelm: Environmentally and animal-friendly keeping of farm, pet and companion animals. Georg Thieme Verlag, 2002, ISBN 3-8263-3139-7 .
- ↑ Jan Wolter, Frank Mutschmann : Ammonia poisoning. In: K. Gabrisch, P. Zwart (Ed.): Diseases of pets. 6th edition. Schlütersche Verlagsgesellschaft, Hanover 2005, ISBN 3-89993-010-X , p. 917.
- ↑ Ulrich Dämmgen, Lotti Thöni, Ralf Lumpp, Kerstin Gilke, Eva Seitler, Marion Bullinger: Process parameters for the determination of ammonia concentrations in the ambient air - Part 2: Measurements with passive collectors. In: Hazardous substances - cleanliness. Air . 70, No. 9, 2010, ISSN 0949-8036 , pp. 367-372.
- ↑ Ulrich Dämmgen, Lotti Thöni, Ralf Lumpp, Kerstin Gilke, Eva Seitler, Marion Bullinger: Process parameters for the determination of ammonia concentrations in the ambient air - Part 1: Measurements with Denuders. In: Hazardous substances - cleanliness. Air . 70, No. 5, 2010, ISSN 0949-8036 , pp. 197-201.
- ↑ VDI 3869 sheet 3: 2010-10 Measurement of ammonia in the outside air; Sampling with coated diffusion separators (denuders); Photometric or ion chromatographic analysis (Measurement of ammonia in ambient air; Sampling with diffusion separators (denuders); Photometric or ion chromatographic analysis). Beuth Verlag, Berlin, p. 17.
- ↑ VDI 3869 sheet 4: 2012-03 Measurement of ammonia in the outside air; Sampling with passive samplers; Photometric or ion chromatographic analysis (Measurement of ammonia in ambient air; Sampling with diffusive samplers; Photometric or ion chromatographic analysis). Beuth Verlag, Berlin, p. 9.
- ^ Franz Joseph Dreyhaupt (ed.): VDI-Lexikon Umwelttechnik. VDI-Verlag Düsseldorf 1994, ISBN 3-18-400891-6 , pp. 637-638.
- ↑ VDI 2461 sheet 2: 1976-05 measurement of gaseous immissions; Measuring ammonia concentration; NESSLER method. VDI-Verlag, Düsseldorf, p. 2.

























![{\ mathrm {4 \ NH_ {3} +5 \ O_ {2} \ {\ xrightarrow [{}] {[catalyst]}} \ 4 \ NO + 6 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5b46496ad526df3502b12b8f4052d4af2a081d71)

![{\ displaystyle \ mathrm {M ^ {II} (NH_ {2}) _ {2} \ {\ xrightarrow [{}] {\ Delta T}} \ M ^ {II} NH \ + \ NH_ {3}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/b9e8e77e2d98999fb572ed5e74b3a46fe690647d)
![{\ mathrm {3 \ M ^ {{II}} NH \ {\ xrightarrow [{}] {\ Delta T}} \ M_ {3} ^ {II}} N_ {2} \ + \ NH_ {3} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b3168ae692d31b78cb78d5e4f24b53fd2edc72ee)

![{\ mathrm {[M (NH_ {3})}} _ {m}] ^ {{n +}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/21c5e7d77669b763733977118ea5b5080a662921)
![{\ mathrm {[Cr (NH_ {3})}} _ {6}] ^ {{3+}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f8016363aedb41fdca29c47e4ed0065f9fae2202)
![{\ mathrm {[Cr (NH_ {3})}} _ {{6-n}} {\ mathrm {L}} _ {n}] ^ {{(3-n) +}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0a01266103772a5dc3a71965f4f43bea339a2ec1)



![{\ displaystyle \ mathrm {NH_ {3} +2 \ K_ {2} [HgI_ {4}] + 3 \ NaOH \ longrightarrow [Hg_ {2} N] I \ downarrow +4 \ KI + 3 \ NaI + 3 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d3067307c0a8b7971a522d969bf5e7499d34a6b6)