molecule
Molecules [ moleˈkyːl ] (older also: Molecules [ moˈleːkəl ]; from Latin molecular molecules , "small mass") are, in the broad sense of the word , two- or polyatomic particles that are held together by chemical bonds and are stable at least long enough that B. can be observed spectroscopically . A molecule can consist of several identical or different atoms. It can be neutral particles, but also radicals , ions or ionic adducts. So are z. B. many types of interstellar moleculesnot stable under terrestrial conditions. IUPAC calls such particles molecular entities .
Basics
In the narrow sense and in the general usage of chemistry, molecules are electrically neutral particles made up of two or more atoms . The atoms are covalently linked to one another and form a self-contained, chemically saturated association. A molecule defined in this way is the smallest particle of a certain pure substance and has a determinable molecular mass . A molecule is not a rigid structure; different molecular vibrations occur when energy is supplied ; the normal temperature is sufficient for this .
Molecules can be made up of atoms of a single chemical element , such as oxygen (O 2 ) and nitrogen (N 2 ) ( elemental molecules ). However, many molecules are associations of atoms of different elements, such as water (H 2 O) and methane (CH 4 ). The arrangement of the atoms (their constitution ) in a molecule is fixed by the chemical bonds. Despite the same number of atoms involved, ethanol (H 3 C – CH 2 –OH) differs from dimethyl ether (H 3 C – O – CH 3 ); they are represented by different chemical formulas . In certain cases, molecules such as B. the molecules of lactic acid forms with the same constitution, but different spatial arrangement ( configuration ). The fact that the same empirical formulas allow different molecules is generally called isomerism .
A single molecule has the chemical properties of a substance. The physical properties such as the boiling or melting point of a molecular substance are determined by intermolecular forces and can lead to the formation of molecular lattices in the case of solids . Large molecules are called macromolecules . Plastics such as PET and biopolymers such as starch are made up of macromolecules .
The size of diatomic molecules is in the range of 10 −10 m (1 Å ), relatively large molecules made up of a large number of atoms reach a diameter in the range of 10 −9 m (10 Å), whereby macromolecules can be slightly larger. Experimentally, the size of molecules z. B. estimate with the oil stain test .
The bonding relationships in molecules are explained and described , for example, with the VSEPR model or the MO theory .
Demarcation
Not all chemical compounds are made up of individual molecules. No molecules lie e.g. B. with diamond-like substances such as boron carbide (B 4 C) and silicon carbide (SiC). The atoms are held together by covalent bonds , but a typical molecule cannot be determined. The chemical formula is just a ratio formula . The arrangement of the atoms can be represented by a unit cell , which repeats itself over and over again and ends with formally open (unused) valence electrons on its surface.
No molecules are present in salts such as sodium chloride (NaCl), which are held together by ionic bonds . Here, too, the formula shows the ratio of the atoms involved, and here too the association of atoms can in principle have any size and reach a range of a few millimeters. The basic elements of this type of compound are particles (here atoms) with a charge . Such particles are commonly called ions . The sodium atom forms a cation (Na + ), the chlorine atom an anion (Cl - ). In the case of sodium sulphate (Na 2 SO 4 ), the SO 4 2− anion consists of a group of atoms that carries a charge. Atomic compounds with charges are not molecules in the strict sense . This is also common in organic chemistry: acetic acid consists of molecules, the anion of the acid is called the acetate ion . A special case is mass spectrometry , in which the term molecular ion is used.
Polyatomic radicals are also not molecules in the narrower sense , since these particles are not chemically saturated. It is enough and unequivocal to call them radicals. In organic chemistry in particular , they are highly reactive intermediates in certain chemical reactions . However, there are also stable radicals such as nitric oxide or TEMPO . Here intermolecular forces lead to physical properties of the connections and these connections can be considered as molecular.
Modes of representation
There are different ways to represent molecules. Basically, a distinction can be made between formulas, two-dimensional schemes (so-called structural formulas) and three-dimensional models (so-called stereo formulas). Sum formulas have the lowest, stereo formulas the highest information content . In order to be able to infer the actual arrangement from abstract spellings, one should be aware that the covalent bonds are arranged roughly in the form of a tetrahedron . (see covalent bonds; spatial alignment )
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |

|

|
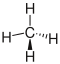
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
Common spatial molecular models are the space- filling model and the rod model .
Web links
See also
Individual evidence
- ↑ Entry on molecular entity . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03986 .
- ↑ Entry on molecule . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M04002 .
- ↑ Der Brockhaus, Science and Technology , FA Brockhaus, Mannheim; Spectrum Academic Publishing House, Heidelberg, 2003.
- ↑ Entry on molecules. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Entry on molecular ion . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03988 .