plastic
As plastics (including plastic , rarely tech polymers , commonly known as plastic ) are materials referred mainly of macromolecules exist.
Important characteristics of plastics are their technical properties, such as malleability, hardness , elasticity , breaking strength , temperature and heat resistance and chemical resistance , which can be varied within wide limits by the choice of macromolecules, manufacturing processes and usually by adding additives . With regard to their physical properties, plastics are divided into three large groups: thermoplastics , thermosets and elastomers .
Plastics are processed into molded parts, semi-finished products , fibers or films . They serve as packaging materials , textile fibers , thermal insulation , pipes, floor coverings , components of paints , adhesives and cosmetics , in electrical engineering as a material for insulation , circuit boards , housings, in vehicle construction as a material for tires , upholstery , dashboards , petrol tanks and much more.
The respective macromolecules of a plastic are polymers and are therefore made up of repeating basic units . The size of the macromolecules of a polymer varies between a few thousand to over a million basic units. For example, the polymer polypropylene ( abbreviation PP) consists of repeatedly repeating propylene units. The polymers can be straight, branched or crosslinked molecules.
The polymers can be obtained from natural substances or be purely synthetic. Synthetic polymers are produced from monomers or prepolymers by chain polymerization , polyaddition or polycondensation . Semi-synthetic plastics are produced by modifying natural polymers (mainly cellulose to celluloid ), while other bio-based plastics such as polylactic acid or polyhydroxybutyric acid are produced by fermenting sugar or starch.
Between 1950 and 2015, around 8.3 billion tons of plastic were produced worldwide - this equates to around 1 ton per capita of the world population. Half of the production comes from the last 13 years. Of this amount, approx. 6.3 billion tonnes were turned into waste , 9% of which was recycled , 12% incinerated and 79% landfilled on landfills or accumulated in the environment. Plastics in general have been criticized for their waste issues and potential health hazards .
Development history of plastics
Prepress
Biopolymers and naturally occurring polymers have been used by humans since ancient times. All animals and plants contain polymers in their cells. Wood initially served people as firewood and tools , such as throwing wood , spears and building material . The cell structure of animal skin or fur was stabilized by tanning , thus protecting it from rapid decay and thus becoming durable leather . From wool , cut off animal hair, clothing and blankets were made by spinning and weaving or by felting .
Birch trees provided the first plastic in human history, birch pitch obtained from birch bark by dry distillation , which was used by both Neanderthals and the Stone Age Homo sapiens as an adhesive in the manufacture of tools.
In Mesopotamia, water basins and canals were sealed with natural asphalt . Certain tree resins were also used there as gum arabic and exported to Europe. Amber is known from Europe as a fossil resin for use in arrowheads and jewelry. In the Middle Ages, animal horn was transformed into a plastically deformable material through certain process steps. As early as 1530, the Fugger's house was producing and selling transparent artificial horn from goat cheese based on a recipe by the Bavarian Benedictine monk Wolfgang Seidel .
Development of a plastics industry
Early discoveries
In the 17th and 18th centuries, naturalists brought elastic masses ( rubber ) obtained from milky tree sap from Malaysia and Brazil . The term rubber was introduced for these in Germany . A rapidly growing rubber industry developed since the middle of the 19th century.
The inventor Charles Goodyear discovered in 1839 that rubber changes into rubber when exposed to heat by adding sulfur . This process is called vulcanization . Charles Goodyear first made rubber gloves from the new material. Around 1850 he also discovered hard rubber , a natural rubber hardened by heating in the presence of sulfur , which was initially marketed as ebonite . From this, for example, jewelry, fountain pens, piano keys, tobacco pipes and parts of telephones were made. This first duroplast started the development of plastics as a material in the human environment.
The development of the celluloid is thanks to several chemists. Christian Friedrich Schönbein developed gun cotton in 1846 by adding nitric acid to cotton. The Englishman Maynard dissolved gun cotton in an ethanol-ether mixture and obtained elastic membranes ( collodion ) after evaporation . The Englishman Cuttin kneaded the collodion with alcoholic camphor solution to form celluloid. In 1869, John Wesley Hyatt used celluloid as a plastic and three years later developed the first injection molding machine . Cellulose nitrate for the impregnation of textiles was developed in England and shellac in the USA .
Linoleum was invented by Frederic Walton in 1844. It was obtained from linseed oil , siccatives and resins by blowing air. Areas of application were floor coverings, wall coverings, table surfaces.
Max Fremery and Johann Urban dissolved cellulose with an ammoniacal copper hydroxide solution. With this solution ( Cupro ) it was easy to produce copper-rayon threads as the first viscose fiber .

In 1872, Adolf von Baeyer described the polycondensation of phenol and formaldehyde . The Belgian chemist Leo Hendrik Baekeland investigated the effect of acid and alkali in this reaction and in 1907 developed a process (in technical production since 1909) for the production and further processing of a phenolic resin . This plastic, which he baptized Bakelite, was the first synthetic thermoset to be industrially produced in large quantities. Thanks to its suitability as an electrical insulator, it was used, among other things, in the emerging electrical industry.
Wilhelm Krische and Adolf Spittler developed the Galalith ( artificial horn ) in 1885 . The plastic is very similar to animal horn or ivory . The artificial horn is made from casein and formaldehyde solution. For example, buttons, pins, housings for radios, cigarette boxes, toys, and handles for umbrellas in various colors were made from it.
In 1909, the German chemist Fritz Hofmann registered a patent for the synthetic rubber Buna . The first fully synthetic tires made from isoprene rubber were produced in 1912.
The Berlin pharmacist Eduard Simon described polystyrene in 1839 . The styrene initially turned into a gelatinous mass. In 1909, H. Stobbe studied the polymerization reaction of styrene in detail. This discovery was not used until twenty years later.
In 1835 Victor Regnault discovered vinyl chloride, from which polyvinyl chloride (PVC) could be made. The first patenting of PVC and polymers made from vinyl acetate goes back to Fritz Klatte in 1912. However, Coroplast is a global pioneer in plastics processing and was one of the first companies to process PVC. It was not until 1950 that this process was replaced by improvements made by Dow Chemical .
As early as 1901 Otto Röhm was engaged in the production of acrylic acid and acrylic acid esters. But it wasn't until 1928 that he found the methyl methacrylate that was better suited for polymerization. The patent for polymethyl methacrylate (PMMA, trade name “Plexiglas”) started an era.
Development of polymer chemistry
Until the late 19th century, little was known about the exact structures of polymeric materials. It was only known from vapor pressure and osmosis measurements that very large molecules with a high molar mass were involved . It was wrongly believed that these were colloidal structures.
The German chemist Hermann Staudinger is considered the father of polymer chemistry . As early as 1917, he said to the Swiss Chemical Society that “high molecular compounds” consist of covalently bound, long-chain molecules. In 1920 he published an article in the reports of the German Chemical Society , which is considered to be the foundation of modern polymer science. In the years from 1924 to 1928 in particular, other important theories about the structure of plastics followed, which form the basis for today's understanding of this class of materials. Staudinger received the Nobel Prize in 1953 for this work .
Staudinger's work enabled the chemical industry to develop rapidly in the field of polymer chemistry based on established scientific principles.
In 1910, the Munich chemist Ernst Richard Escales named the group of materials "Kunststoffe". The magazine of the same name, which he founded, first appeared in 1911.
At Imperial Chemical Industries (ICI) in Great Britain, polyethylene was first produced under high pressure (200 bar) and at high temperatures in 1933 . It was not until twenty years later that Karl Ziegler developed a process that, using catalysts made from aluminum alkyls and titanium tetrachloride, allowed the polymerization of ethene to form polyethylene at room temperature. The low-pressure polyethylene proved to be more heat-stable and more mechanically resilient. Shortly afterwards, Ziegler and Giulio Natta found a catalyst for the polymerization of propene to polypropylene . In 1955–1957 the large-scale syntheses of polyethylene and polypropylene began. Today, the polyethylenes (PE) and polypropylene (PP) produced in this way are, along with polystyrene (PS), the plastics most frequently used as packaging materials for food, cosmetics, etc. Ziegler and Natta received the Nobel Prize in Chemistry in 1963 for their work.
Plastics made from polyesters were considered very early on (Berzelius, 1847). In 1901 there were glyptal resins (made from glycerine and phthalic acid). Fritz Hofmann, Wallace Hume Carothers and Paul Schlack searched unsuccessfully for synthetic fibers based on polyesters. It was not until the British Whinfield and Dickson that Calico Printers succeeded in manufacturing usable polyester fibers (polyethylene terephthalate, PET) in 1941. Important polyester fibers were Dacron (DuPont), Diolen ( ENKA-Glanzstoff ), Terylen (ICI), Trevira (Hoechst).
In 1934, the production of epoxy resins began in Ludwigshafen using a process developed by Paul Schlack. In 1935, Henkel (Mainkur) and Ciba (Switzerland) described the development of melamine resin at the same time .
In 1931, the US chemist Wallace Hume Carothers applied for a patent to DuPont for a polyamide made from hexamethylenediamine and adipic acid . The new synthetic fiber nylon (1938) was only marketable seven years later . The polyamide 6 based on caprolactam produced by Paul Schlack in 1937 was christened Perlon . Large-scale production began in 1939 at IG-Farben . The manufacturing process of Perlon in Germany was cheaper than the nylon production in the USA.
The about the same time began Buna works of IG Farben with the production of Buna S and Buna N as synthetic rubber substitute. In 1939 Otto Bayer developed polyurethane (PU) in Leverkusen.
At DuPont, the plastic polytetrafluoroethylene (Teflon) was developed by RJ Plunkett in 1938 . The product showed high temperature resistance and high chemical resistance. However, processing encountered problems. Teflon did not go into large-scale production until 1946.
Silicone had already in 1901 Frederick Kipping made of silanones. It was only through the synthesis of organosilicon halides with alkyl halides that silicone could be produced cheaply in the USA and Germany in 1944 ( Eugene G. Rochow , Richard Müller ).
The polymerization of acrylonitrile had been known since the early 1930s . However, it was not usable as a plastic. The chemist Rein was able to dissolve polyacrylonitrile in dimethylformamide and thus make it useful for plastics production. In 1942, IG Farben developed a polymerisation process to produce polyacrylonitrile. In 1942 Harry Coover (USA) discovered the “ superglue ” methyl cyanoacrylate at Eastman Kodak .
Classification
Depending on the perspective of the observer and the requirements, plastics can be classified in different ways. Classifications according to mechanical-thermal behavior (most common classification), origin (natural or synthetic), use or development reaction are common. A strict demarcation of individual plastics is often not possible, but these classifications offer a good overview.
Classification according to mechanical-thermal behavior
The mechanical-thermal behavior is classified into thermoplastics, thermosets and elastomers. In addition, there are thermoplastic elastomers and reversible thermosets , which are clearly of minor importance . This classification is of application engineering origin. The different polymer classes differ in their mechanical properties due to the different cross-linking and the respective relationship between usage temperature (mostly room temperature ) and physical transition temperature ( glass transition temperature and melting point ).
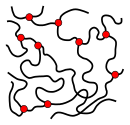
Thermosets consist of closely meshed, cross-linked polymers. Networks are shown in the figure as red dots. Elastomers consist of wide-meshed cross-linked polymers. The wide mesh allows the material to stretch under tensile stress. Thermoplastics consist of uncrosslinked polymers, often with a partially crystalline structure (shown in red). They have a glass transition temperature and are fusible.
Thermoplastics
Thermoplastics are plastics that consist of long linear molecules. By supplying energy, these materials become soft and malleable ( plastic ) any number of times and finally melt. They can be brought into the desired shape using various primary and forming processes . After the workpiece has cooled, it retains its shape. This process is thus reversible (lat. Reversible). This behavior is caused by thread-like, linear macromolecules.
Most of the plastics used today fall under this group (polyethylene, polypropylene, polystyrene, polyester). They are just as frequently used for simple consumer goods, packaging, etc. as they are for technical parts in the automotive and electrical industries or in the construction industry, especially for roofing membranes, window profiles and pipes.
In order to create new properties that have not yet existed, two or more (mutually compatible) thermoplastics can be mixed ( polyblend ).
Semi-crystalline thermoplastics (examples): POM - polyoxymethylene, PE - polyethylene, PP - polypropylene, PA - polyamide, PET - polyethylene terephthalate, PBT - polybutylene terephthalate.
Amorphous thermoplastics (examples): ABS - acrylonitrile-butadiene-styrene, PMMA - polymethyl methacrylate, PS - polystyrene, PVC - polyvinyl chloride, PC - polycarbonate, SAN - styrene-acrylonitrile copolymer, PPE - polyphenylene ether.
Thermosetting plastics

Duroplasts (thermosets) are polymers that are produced in a curing process from a melt or solution of the components through a crosslinking reaction. This irreversible reaction is usually brought about by heating (hence the technical term thermosets ), but it can also be initiated and accelerated by oxidizing agents, high-energy radiation or the use of catalysts. Heating thermosetting plastics does not lead to plastic deformability, but only to their decomposition. Hardened thermosets are usually hard and brittle and can only be processed mechanically in the further manufacturing process. The reason for this behavior are the networked macromolecules.
Because of their mechanical and chemical resistance, even at elevated temperatures, they are often used for electrical installations . The most common and oldest type of plastic in this class are phenoplasts . This group also includes polyester resins, polyurethane resins for paints and surface coatings and practically all synthetic resins such as epoxy resins .
Elastomers
Elastomers can change their shape briefly through pressure or stretching; after the end of pressure or stretching, the elastomer quickly returns to its original shape. The elastomers are wide-meshed and therefore flexible. They do not soften when heated and are not soluble in most solvents.
Elastomers include all types of crosslinked rubber . The crosslinking takes place, for example, by vulcanization with sulfur, by means of peroxides , metal oxides or irradiation . 60% of elastomers are used for tires . The rest is distributed among other rubber items, for example chemical gloves and hygiene articles.
Elastomers are natural rubber (NR), acrylonitrile butadiene rubber (NBR), styrene butadiene rubber (SBR), chloroprene rubber (CR), butadiene rubber (BR) and ethylene propylene diene rubber (EPDM).
Classification according to origin
From a chemical point of view, plastics as macromolecular substances can be compared with other macromolecular substances. The different macromolecular substances can then be classified according to their origin:
- natural macromolecular substances such as hydrocarbons (rubber, balata), polysaccharides (cellulose, starch, pectin, chitin, cotton) and proteins (collagen, wool, silk)
- Derivatives of natural macromolecular substances such as cellulose nitrate , leather or gelatine
- synthetic macromolecular substances
- Derivatives of synthetic polymers (modification for example by saponification, introduction of reactive groups or subsequent crosslinking)
Only some of the macromolecular substances listed are plastics in the narrower sense, since plastics are defined as substances that are based on polymers and also go through "plastic" states as materials during processing. Nevertheless, this classification can contribute to understanding.
Classification according to application
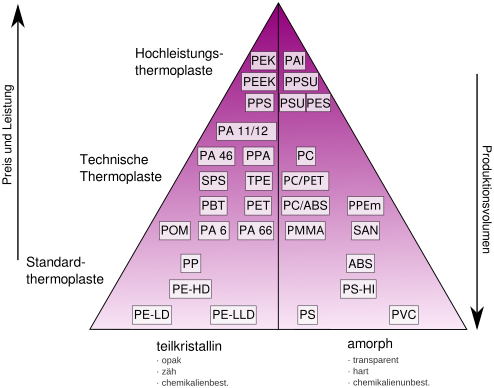
Depending on the price, volume and possible use of thermoplastics can be divided into four classes of applications: Standard plastics , engineering plastics , functional plastics and high-performance plastics . Standard plastics (also: mass plastics) are very versatile and are produced in large quantities. Standard plastics are often used as packaging material, for example polyethene or polyvinyl chloride . Engineering plastics have better mechanical properties than standard plastics and retain them above 100 ° C and below 0 ° C. Engineering plastics are widely used for engineering constructions, including polyethylene terephthalate and some aliphatic polyamides . Functional plastics only serve a single function, for example as a barrier for flavors and gases in plastic packaging. Thermosets cannot be classified according to this scheme, but form a class of their own.
Compared to standard, technical and special plastics, high-performance plastics are distinguished by their heat resistance and z. T. also have good mechanical properties. While the heat resistance of standard plastics is usually only around 100 ° C and that of engineering plastics reaches up to 150 ° C, high-performance thermoplastics can withstand temperatures of up to 300 ° C. High-performance plastics are quite expensive at around € 20 per kg ; their market share is only around 1%.
The comparison of standard plastics, engineering plastics and high-performance plastics is illustrated by the following figure:
Classification according to development reaction
Plastics are produced through various polyreactions : polymerisation , polycondensation and polyaddition . Accordingly, the product is referred to either as a polymer, as a polycondensate or as a polyadduct.
International abbreviation system
Individual plastics are designated according to a globally standardized system of abbreviations , which is defined for Germany in DIN EN ISO 1043 Part 1: 2016-09: Basic polymers and their special properties , DIN ISO 1629: 2015-03: Rubber and latices - nomenclature (ISO 1629: 2013) and DIN EN ISO 18064: 2015-03: Thermoplastic elastomers - nomenclature and abbreviations (ISO 18064: 2014; German version EN ISO 18064: 2014) .
properties
Compared to ceramic or metallic materials , plastics are characterized by a number of unusual properties:
Density and strength
The density of most plastics is between 0.8 and 2.2 g · cm −3 . It is therefore lower than that of metallic (from Mg 1.8 to Cu 8.5 g · cm −3 ) or ceramic materials (from approx. 2.2 to 6 g · cm −3 ).
In terms of mechanical properties, plastics are often inferior to other material classes. Their strength and rigidity usually do not match that of metals or ceramics . Due to the low density, however, this can be partially compensated for with structural means (higher wall thicknesses) or the use of fiber-reinforced plastics .
Although the strengths are comparatively low, plastic parts break less easily than, for example, ceramic or glass due to their mostly good toughness . Therefore, everyday objects for children and toys are often made of plastic.
Chemical resistance
In contrast to metals, many plastics are resistant to inorganic media due to their organic nature . This includes mineral acids , alkalis and aqueous salt solutions . Therefore, materials made of plastic were preferred for the production of easy-care household and electrical appliances, vehicle equipment, toys, etc.
In contrast to metals, however, they are sensitive to organic solvents such as alcohol, acetone or gasoline. Nevertheless, it was also possible to develop durable plastics in this area. One example is the polyethylene fuel tank in modern passenger cars, which is extremely resistant to corrosion and yet insensitive to gasoline.
Degradation in plastics
In plastics, degradation refers to their degradation or disintegration. One speaks more often of “ aging ” and this usually results in swelling , embrittlement , cracking and loss of strength . The degradation is usually an undesirable process and takes place either chemically, physically or through a combination of both types of degradation.
Low processing temperatures
The usual processing temperatures for plastics are in the range from 250 to 300 ° C. While metals have to be cast in a complex manner at high temperatures and there are restrictions with regard to the casting molds, more complicated molded parts can be manufactured from thermoplastics with comparatively little effort (see extrusion and injection molding ). At the same time, additives such as color pigments or fibers can be incorporated into the material in one processing step , which would decompose at the high temperatures of metal casting or sintering of ceramics.
Low conductivities
The thermal conductivity of plastics is only a fraction of that of metals. Since, for this reason, comparatively little heat energy is transferred from the hand when touched (so plastics still feel warm at low temperatures), handles on tools or railings are often made of plastic or covered with it.
Materials such as foams, fleeces and flakes isolate primarily through the content of (spatially fixed) air. Plastics as matrix material promote the insulating effect; such as in insulation boards, textiles or mattresses . The easy flammability, however, is a clear disadvantage compared to mineral glass or rock wool, sheep and cotton , cork, but also solid wood .
The electrical conductivity of plastics is 15 orders of magnitude less than that of metals. Therefore, plastics are used for insulation . Metallized plastic foils are used as a dielectric and rolled up to form capacitors . The high surface resistance, which leads to electrostatic charging with friction via contact electricity, can be broken with fillers (such as in shoe soles) or antistatic agents in furniture polish or textile detergents.
Manufacturing
Plastics are generally produced by gradually joining monomers together to form long chains - the polymers - whereby a basic distinction is made between chain polymerization and step polymerization .
Chain polymerizations
In chain polymerization, growth begins with a molecule to which further monomers are successively added. The molecule that starts the polymerization is called the initiator , the one growing on top of it is called the monomer . The number of monomers that ultimately make up the polymer is the degree of polymerization . The degree of polymerization can be adjusted through the ratio of monomer to initiator. Mathematically, it is estimated by the Mayo equation .
Free radical polymerization
In radical polymerization, the growth reactions are initiated and propagated by radicals . Compared to other chain reactions, it is insensitive, easy to control and delivers high degrees of polymerization even with very small conversions. It is therefore mainly used in the production of cheap plastics such as LD-PE, PS or PVC.
A danger in this process is the heat of polymerization that is released. Free-radical polymerization is exothermic , i.e. heat is released during the reaction. If not dissipated, this heat generates further radicals so that the reaction can accelerate itself. In extreme cases, such “self-acceleration” can overload the reactor material and thus lead to a thermal explosion .
Ionic polymerization
In ionic polymerizations, the growth reactions are initiated and propagated by ionic species. The growing chains are more durable (several hours to days) than their radical analogues (lifespan about 10 −3 s). Living polymers are also used in this context . Therefore, after a polymerization has ended, the chains that are still alive , i.e. those capable of polymerization, can be given up on another monomer and renewed growth can be stimulated.
Polymers whose chains consist of two or more different types of monomers are called copolymers . If there are long blocks of one monomer in a copolymer followed by blocks of the other, these are block copolymers . Ionic polymerization is used for such special applications. One example is the synthetic rubbers acrylonitrile butadiene rubber (NBR) and styrene butadiene rubber (SBR), which are used in the manufacture of car tires. The disadvantage of this process is its high sensitivity to impurities, water and oxygen. Ionic polymerizations are therefore more complex and cost-intensive than radical polymerization.
Organometallic catalysts
These polymerizations take place in the presence of catalysts. The catalyst is a metal complex (compound of metal atoms surrounded by other species) that is able to bind the growing chain. The addition of further monomers is done by insertion (insertion) of the monomer between the growing chain and catalyst species. The result is a higher degree of order of the resulting polymers and a lower degree of branching. Due to this more regular structure, the individual chains are packed more efficiently in the solid, and the plastic becomes more dense . The most important class of catalysts in industry at the moment is that of the Ziegler-Natta catalysts . They play a role, for example, in the manufacture of polyethylene.
Low-density polyethylene (LD-PE) is ethene polymerized in the gas phase with a low degree of order, many side branches and low density. This plastic is used as a transparent or colored packaging film for beverage bottles, books and CDs.
High-density polyethylene is produced with an organometallic catalyst in the Ziegler-Natta process . The result is a polymer with a high degree of order, few branches and high density. This plastic is used, for example, as a material for car tanks, petrol cans, etc.
Step polymerizations
In contrast to chain polymerizations, the formation of the polymers in step polymerizations does not take place through the initiation of a growing chain, which further adds successive monomers, but through direct reaction of the monomers with one another. This reaction can take place with the release of a by-product such as water as a polycondensation or by simply adding the monomers to form a new compound by polyaddition.
Polycondensation
In the case of polycondensations , the linear chain is formed by an intermolecular reaction of bifunctional polymers with the elimination of a smaller species, such as water or alcohols. Polycondensation is of major importance for polyamides .
Carboxylic acids react with amines to form amides . If molecules are used that carry two carboxylic acid groups, one of these molecules can react with two amines. The result is a polymer made up of three monomers (one carboxylic acid unit, two amines). If the amines used also carry two amine groups again, the previously created species can in turn react with two carboxylic acid molecules. The resulting polymers can bond with each other even further, so that the degree of polymerization depends crucially on the duration of the reaction. The process is described by the Carothers equation .
Polyesters are produced by reacting dicarboxylic acids with diols (dialcohol). Among the most important plastics made by polycondensation are polyesters such as polyethylene terephthalate (PET), polyamides and phenoplasts . Maleic acid and phthalic acid polyesters are produced industrially from their anhydrides.
Polyaddition
In the case of polyadditions , the polymer is formed by adding the individual monomers to one another without the formation of by-products. A large group of polyadducts form the polyurethanes .
Isocyanates react with alcohols in an addition reaction to form so-called urethanes. The same applies here: bifunctional monomers form long linear chains. Polyurethane produced in this way is used for dashboards, paints, adhesives, etc. If water is added to the polymerization mixture, it reacts with the isocyanates to form ureas and carbon dioxide. The CO 2 released in the mixture is enclosed in the plastic in the form of bubbles and a foam is created. Polyurethane foam is used for mattresses, seating, sponges, etc.
Additives
So-called additives are added to plastics during the manufacturing process ( compounding ) . They are used to precisely adjust the material properties to the needs of the respective application and to improve the chemical, electrical and mechanical properties. Such molding compounds provided with additives are labeled in accordance with DIN EN ISO 1043 (thermoplastics) and DIN 7708 (thermosets).
Plasticizers
Around two thirds of the additives manufactured worldwide are used in the production of polyvinyl chloride, and almost three fifths of the additives manufactured are plasticizers . They reduce the brittleness , hardness and glass transition temperature of a plastic and make it easier to shape and process. These are substances that are able to penetrate the plastic on a molecular level and thus increase the mobility of the chains against each other. Qualitatively, they can serve as a “molecular lubricant”. Until a few years ago, diethylhexyl phthalate (DEHP) (synonym: dioctyl phthalate DOP) was the most frequently used plasticizer. However, this turned out to be harmful to the environment and health, which is why European industry now largely wants to forego its use. The 1,2-cyclohexanedicarboxylic acid diisononyl ester (DINCH) introduced in 2002 is often used as a replacement for DEHP . Other new plasticizers are the analogous adipic acid esters such as diethylhexyl adipate .
Extenders also improve processability and are therefore also called secondary plasticizers . Important extenders are epoxidized oils, high-boiling mineral oils and paraffins.
Stabilizers
Stabilizers are used to improve the chemical properties. They increase the service life of the plastic and protect it from damaging influences (oxidation, ( UV ) radiation and heat from fire, for example) in its area of application.
The plastic can discolour through reaction with atmospheric oxygen and the polymer chains can decompose or re-crosslink. This is prevented by adding antioxidants , which intercept the free radicals that arise during the reaction (radical chain terminators) , or prevent the formation of radicals (deactivators) . Phenols or amines , for example, are suitable as terminators, while phosphines and amines serve as deactivators .
Sunscreens protect against damage from ultraviolet light . Double bonds between carbon atoms are able to absorb light of this wavelength, which is why plastics in particular are at risk from UV light that contain this structural element ( e.g. polyisoprene ). However, due to catalyst residues, structural defects and side reactions during processing, practically all polymers can exhibit an absorption capacity for UV radiation. This induces the formation of free radicals in the material, which initiate side reactions such as chain breakdown and crosslinking. There are basically three ways to prevent damage: reflection of light, addition of light-absorbing substances and addition of radical scavengers. Important light stabilizers are carbon black , which absorbs the radiation, σ- hydroxybenzophenone , whichconvertsthe energy into infrared radiation , and dialkyldithiocarbamates, which absorb UV light and act as radical scavengers.
Plastics are sensitive to the effects of heat. Above a temperature that is characteristic of the material (decomposition temperature) , the breakdown of the molecular structure begins. Heat stabilizers are designed to prevent this. These are essential for polyvinyl chloride, which otherwise, with the formation of hydrogen chloride and u. U. health-damaging decomposition products, would lose its mechanical stability. The disintegration mechanism takes place via the formation of double bonds . Organic barium, zinc, tin and cadmium compounds and inorganic lead salts complex these and thus interrupt the disintegration mechanism. In particular, the lead compounds represent a not inconsiderable environmental problem with regard to the disposal of the plastic. Currently 80% of the heat stabilizers are based on lead. However, the chemical industry is currently trying to replace them. At Cognis, a stabilizer based on calcium and zinc has been specially developed for window profiles.
Plastics can be dangerous in the event of a fire, as many plastics provide food for a fire and some plastics release toxic or corrosive gases (such as hydrogen cyanide , carbon monoxide , hydrogen chloride or dioxins ) when burned . Flame retardants either prevent oxygen from entering the fire or disrupt the chemical reactions (radical chain mechanisms) of the combustion.
Important flame retardants are:
- polybrominated diphenyl ethers (PBDE): set free radicals which intercept the intermediate products of the burning process
- Aluminum hydroxide (Al (OH) 3 ), also (ATH): releases water molecules
- Phosphorus-containing compounds: form phosphoric acids that catalyze the elimination of water
Colorants
Most polymers are colorless in their pure form; they only become colored when colorants are added . A distinction must be made between dyes (dissolve at the molecular level in the polymer or adsorb on the surface) and pigments (insoluble, mostly organic / inorganic aggregates). Textiles are almost exclusively colored with dyes. However, the vast majority of plastics are colored with pigments, as these are more lightfast and usually cheaper. Important pigments in this area are rutile (white), soot (black), cobalt or ultramarine blue , and chrome oxide green . The use of effect pigments is now possible; strontium aluminates doped with rare earths show an intense glow at night. Areas of application for such colored plastics are safety markings, light switches or flashlights that are easier to find in the dark. In order to achieve a metallic luster, aluminum pigments in the form of flakes are used; spherical pigment grains produce a gray color. In plastics processing, concentrated pigment preparations, so-called liquid colors or masterbatches, are usually used for coloring .
Fillers
Fillers are classic extenders that make the production of plastic cheaper. “Active fillers” also improve the mechanical properties of the material. Important fillers include: chalk , sand , kieselguhr , glass fibers and spheres, zinc oxide , quartz , wood flour , starch , graphite , carbon black and talc . Fillers are also important to minimize the fire behavior of the plastics.
Soot is of particular importance (car tires, foils, roofing membranes ). Soot is chemically resistant and therefore weather-resistant. As a coloring substance, soot is stable to light and does not fade. Due to its high level of UV absorption, soot provides UV protection for the plastic. This protects the underlying molecular chains in the structure from being destroyed by UV light.
Reinforcement fabrics
Reinforcement materials are additives used in plastics that strengthen the plastic matrix. The result is an improvement in mechanical and physical properties such as elasticity or flexural strength. Examples are glass fibers, carbon fibers or flax and jute.
Coating
Coating with metals is called plastic metallization . It is used in areas in which plastic is used to replace metals, but where the higher quality appearance of metallic luster is to be retained. In the automotive industry, galvanized plastic elements are used in exterior cladding. In electrical appliances, the metallized plastic allows shielding. Elements for mixer taps, shower heads and faucet handles are used in the sanitary area.
Plastics industry
The plastics manufacturing industry is an important branch of the chemical industry . There are partially overlapping areas
- Plastics production (production)
- Plastics processing
- Plastics engineering
production
Especially after 1950, the production of plastics increased enormously due to the numerous successes in the field of polymer chemistry. Thanks to the development of thermoplastics and, in particular, corresponding processing methods, molded parts could now be produced in an unbeatably cheap way. Plastic went from being a substitute material of particular importance to being a material for industrial mass production. As a result, the proportion of thermosets fell steadily and in 2000 was only 15%. The per capita consumption of plastics in 2000 was 92 kg in Western Europe, 13 kg in Eastern Europe, 130 kg in North America , 19 kg in Latin America, 86 kg in Japan, 13 kg in Southeast Asia and 8 kg in the Middle East / Africa .
The plastics industry is still a growth sector today, with manufacturing capacities in Asia overtaking the leading and roughly equally strong regions of Europe and North and South America between 2006 and 2008. In 2006, 3570 companies with around 372,900 employees achieved total sales of 79.4 billion euros in this area in Germany. The global production of plastics is largely carried out by global chemical groups such as Asahi Kasei , Basell , BASF , Bayer , Celanese / Ticona , DuPont de Nemours , DSM , and Solvay . They supply a limited range of plastics in quantities of sometimes several hundred kt per year. The prices for plastics vary widely from a few euro cents per kilogram for bulk plastics to a few hundred euros per kilogram for high-performance polymers.
processing
Plastics processing is the subject of an independent branch of industry. In this case, mainly come molding method used, which occur in contrast to the metallic materials at significantly lower processing temperatures (430 ° C). As a result, the production facilities (so-called tools ) can be used multiple times and thus allow cost-effective production.
A large number of processes are used, some of which have their origins in much older metalworking and have been adapted and further developed to the properties of plastics. For example, injection molding for plastics is very similar to die casting for metals. The extrusion or blow molding is made of glass production emerged.
The foaming process , in turn, has its origins in plastics, but, like metal foam , is now also used for other classes of materials. They can be further subdivided into chemical, physical or mechanical blowing processes.
For all of these processes, special machines and tools are required that are made available by plastics engineering.
Important bulk plastics
See also the listing under List of plastics
Around 90% of global production (around 350 million tons per year) are made up of the following six plastics in the order of their share:
Polyethylene (PE)
Polyethylene is mainly produced in three different qualities: HD-PE (High-Density-PE) , LLD-PE (Linear-Low-Density-PE) , LD-PE (Low-Density-PE) . HD-PE is synthesized using Ziegler-Natta catalysts; its chains show a very high degree of order and a low degree of branching. These can therefore arrange themselves efficiently in the solid, so that a semi-crystalline material is created, the density of which is higher than that of LD-PE (but both have a density that is lower than that of water). It is used to manufacture bottles, beverage crates, barrels, battery cases, buckets, bowls, etc. LD-PE is polymerized under high pressure in the gas phase, 1-butene , 1-hexene and 1-octene are polymerized into LLD-PE in order to produce a controlled degree of branching. Both variants have a low crystalline content and a high or medium degree of branching. The material has excellent film-forming properties and is mainly used for the production of packaging films for cigarette packets, CDs, books, paper handkerchiefs, etc., as well as carrier bags.

Polypropylene (PP)
Polypropylene is produced almost exclusively by metal catalytic methods, since only the crystalline material obtained in this way has commercially usable properties. It is a very hard, strong and mechanically resilient plastic with the lowest density of all mass-produced plastics. Due to these properties, it has in some cases already replaced metal materials. As with the lid shown on the right, it also shows the so-called film hinge effect , i.e. That is, it can connect the housing and cover to one another through a thin film without breaking due to the bending load. A significant part of the polypropylene produced worldwide is used for food packaging, other areas of application are:
- Automotive industry: as a material for air filter housings, spoilers , headlight housings, seat covers and gas pedals.
- Construction: garden furniture, toilet lids, artificial turf, furniture hinges, etc.
- Miscellaneous: glasses cases, suitcases, school bags, sterilizable medical devices.
Polyvinyl chloride (PVC)
For a long time, polyvinyl chloride was considered to be the most environmentally harmful plastic due to its unusually high chlorine content and the by-products such as chlorine gas and hydrogen chloride (hydrochloric acid) that arise during combustion. In addition, the vinyl chloride required for production is carcinogenic. In general, a distinction is made between hard polyvinyl chloride and soft polyvinyl chloride made with the addition of plasticizers. Rigid PVC is an amorphous thermoplastic and has a high degree of rigidity and hardness. It is extremely flame retardant, but in the heat of an existing fire it can release hydrogen chloride and dioxins. It shows very good resistance to acids, bases, fats, alcohols and oils. For this reason, it is mainly used to manufacture sewage pipes and window profiles. A serious disadvantage is its very low heat resistance, it can only be used permanently up to 65 ° C and briefly up to 75 ° C; and its tendency to "stress whitening" when bending is also disadvantageous. Soft PVC is a rubber-elastic, leather-like thermoplastic. Important applications are the production of floor coverings, seals, hoses, artificial leather, wallpaper, roofing membranes, wood-plastic composite products, etc.
Polystyrene (PS)
Polystyrene is predominantly manufactured as an amorphous thermoplastic, thanks to recent developments there is now also crystalline polystyrene, but this is of less importance. Both variants are characterized by low moisture absorption, good processability and very good electrical properties. They differ in their impact resistance. Disadvantages are its tendency to form stress cracks, its poor heat resistance, flammability and its sensitivity to organic solvents. Polystyrene foamed using carbon dioxide is sold as Styrofoam , among other things .
Application areas:
- Electrical engineering: as insulation of electrical cables, material for housing (as High Impact Polystyrene (HIPS) ), switches, etc.
- Construction industry: as insulating material ( foam polystyrene )
- Packaging: expanded polystyrene, packaging films, yoghurt pots, etc.
Polyurethane (PU / PUR)
The elasticity of the properties of polyurethanes can be varied very widely by choosing the isocyanate- or urethane-containing monomer components. Very elastic PUR textile fibers ( elastane ) are made from polyesters and polyesters containing urethane, and polymers containing urethane are also used as additives in paints and materials for printed circuit boards (Bectron) .
- The best-known application is likely to be polyurethane foams. They are used as mattresses, in car seats, seating, insulating material and sponges. For this purpose, the exact material properties can be set by selecting the individual components.
- The most important application is probably responsible for the rust protection of car bodies. Individual polymers containing hydroxyl groups and urethane groups are deposited on the bare iron bodies. At 120–160 ° C these are then cross-linked with one another, a rust-preventing polymer layer of the same thickness is formed on the iron.
Polyethylene terephthalate (PET)
Polyethylene terephthalate is a polyester made from terephthalic acid and ethylene glycol ; stoichiometric amounts are used in the production process and the esterification is carried out up to a conversion of 99%. The solidified melt crystallizes very slowly, so that amorphous and partially crystalline (C-PET) material can be produced here, depending on the area of application . C-PET has high rigidity, hardness, abrasion resistance and is resistant to diluted acids, oils, fats and alcohols. However, PET bottles are sensitive to hot water.
Application examples:
- Electrical engineering: parts for household and kitchen appliances, computers, etc.
- Mechanical engineering: gears, bearings , screws, springs.
- Vehicle technology: seat belts, truck tarpaulins
- Medicine: implants such as vascular prostheses
Amorphous PET shows lower stiffness and hardness than C-PET, but better impact strength. Because it is transparent but lighter than glass, it is used as a material for beverage bottles and packaging for food and cosmetics. In electrical engineering, PET films are used as a carrier material for magnetic tapes .
Special plastics
Some plastics are produced in large quantities for mass production. Others, on the other hand, are only used in small quantities because their price is high or they are only useful in special applications. Such plastics are called special plastics (English: specialty polymers or special purpose plastics). Some specialty plastics become more common over time and play a role as engineering plastics, while others are reserved for special applications.
Examples of special plastics are high-performance thermoplastics (also called high-temperature plastics), electroactive polymers , polymer electrolytes, liquid crystal polymers , ionic polymers, polymer nanocomposites and others. Some specialty plastics and some more specific applications are presented below.
Plastics for high temperature applications
Thermoplastic plastics that have a continuous service temperature of over 150 ° C are referred to as high-temperature plastics. Since plastics of this type also have special mechanical properties and a special resistance to chemicals , they are also referred to as high-performance plastics. High-performance plastics are expensive and are only produced in small quantities.
Due to their good mechanical properties and a comparatively low density , high-performance plastics are often used as a substitute for metals. The chemical resistance results in further applications. They are therefore used in the aerospace industry (for turbines ), in the automotive industry in hot spots in the engine compartment or in the chemical industry in contact with aggressive chemicals.
Liquid crystalline polymers
Liquid crystalline polymers (engl. Liquid crystalline polymer (LCP)) are called polymers whose chains in the melt liquid crystalline phases form. In crystals there is generally a fixed order, while in liquids and melts the distribution of molecules or atoms is usually largely random. In this respect, the expression liquid-crystalline is actually a contradiction. In LCPs, however, the polymer chains orient themselves parallel to bundles due to intramolecular interactions. For example, aromatic polyamides in sulfuric acid in combination with calcium or lithium chloride form such phases. If such a solution is pressed from a spinneret through an intermediate space with air into a precipitation bath (dry-jet-wet spinning process) , fibers are produced in which the chains are oriented in the direction of the longitudinal axis. Such fibers are able to withstand an unusually high tensile load for plastics, which is comparable to metals or carbon fibers. Due to their low density, they are embedded in synthetic resins ( composites ) and used in aircraft and vehicle construction. Further applications are bulletproof vests, protective helmets, protective suits, surfboards and sailboat construction. Major brands are Kevlar , Nomex and fiber B .
Electrically conductive polymers
Plastics are generally considered to be excellent insulators. This is due to the fact that polymers completely lack the basic requirement for electrical conductivity, quasi free electrons . By adding substances ( doping ) that either add electrons to the chain ( reduction ) or create free spaces for electron movement through removal ( oxidation ) , it is possible to produce electrically conductive polymers . For example, polyacetylene and poly ( p- phenylene) become electrically conductive if they are doped with bromine , iodine or perchloric acid . Other important electrically conductive polymers are polyaniline, doped with hydrochloric acid and polypyrrole from anodic oxidation. Applications are materials for electrodes and battery elements, as well as antistatic coatings. The polymers mentioned above can also be given semiconducting properties by suitable doping . Polymer light-emitting diodes , for example, are made from such materials . The scientists Alan J. Heeger , Alan G. MacDiarmid and Hideki Shirakawa were awarded the Nobel Prize in Chemistry in 2000 for the development of conductive polymers .
Plastics in medicine
Plastics perform a wide range of tasks in medicine: They serve as containers for infusion solutions, components of medical devices, disposable items (syringes, plasters, catheters, tubes, etc.) and implants (heart valves, bone substitutes, joint sockets, resorbable bone screws, etc.). For materials that are in direct or indirect contact with living tissue, special requirements naturally apply: On the one hand, the plastic must not damage the organism, and on the other hand, the biological environment must not impair the material properties of the plastic. If these conditions are met, we speak of biocompatibility . The most important argument for the use of plastics in medicine was and is hygiene, so medical instruments made of glass or metal could be replaced by disposable items made of plastic. A notable example is polylactic acid (also: polylactide ), a polyester of naturally occurring lactic acid . It is spun into fibers that are used as absorbable surgical sutures. After the threads have been used, they are broken down enzymatically. The duration of the degradation can be adjusted via the stereochemistry (choice of chains from dextrorotatory or levorotatory lactic acid) of the polymer.
Environmental issues

The problem of disposing of the products made from them ( plastic waste ) inevitably arises from the production of plastics : the polymeric components of plastics are, on the one hand, not water-soluble and, on the other hand, are not able to pass through the cell membranes of microorganisms, i.e. Interaction with living organisms is not known except for biodegradable plastics and the formation of microplastics . This has the advantage that polymers can be classified as harmless to health, but a transformation in living nature cannot be completely ruled out. Furthermore, many of the additives used to manufacture plastics have been shown to be harmful to health.
The hormonally active substances ( endocrine disruptors ) used for the production of plastics are now so widespread worldwide that endocrine disruptors can be detected in the urine, blood and fatty tissue of practically all people around the world.
The environmental problems caused by plastics are illustrated in the documentary films Plastic Planet (2009) by Austrian director Werner Boats, Plastik über alles (OV: Addicted to plastic ) (2008) by Canadian director Ian Connacher and Midway (2009-2013) by US director Chris Jordan shown.
Components
Components contained in various plastics are classified as hormonally effective (endocrine disruptors) and are absorbed through the skin, through inhalation (aerosols, abrasion from rubber tires) and through human food:
Bisphenol A.
Bisphenols such as bisphenol A (BPA), C (BPC) or S (BPS) are used as hardeners, for example in the coatings of cans or in plastic storage or other containers: they dissolve mainly in acidic substances such as tomato or fruit liquids, and even faster under the influence of heat. According to statistics, 95 to 98% of people have BPA in their urine , with the half-life of its breakdown in the body being around half a day to a day. According to UNEP / WHO and endocrinological societies, bisphenol A increases the risk of obesity, diabetes mellitus, infertility in men and women, breast cancer, endometriosis, prostate cancer, childhood developmental delays and brain damage.
Phthalates
Phthalates are used as plasticizers in cosmetics, but also in food films: however, they are hormonally effective in the sense of an endocrine disruptor and thus increase the risk of obesity, diabetes mellitus, infertility in men and women, and breast cancer, according to UNEP / WHO and endocrine specialist societies , Endometriosis, prostate cancer, childhood developmental delays and brain damage.
entry
In some cases, non-biodegradable plastics also end up in the environment. According to various estimates, six to 26 million tons of the more than 200 million tons of plastics produced annually worldwide end up in the oceans, 70% of which sink to the sea floor. Less than 5% of the total originates from Europe and North America together. In investigations in 42 countries, most of the plastic waste found falls on Coca-Cola , Pepsi and Nestlé . In the Manila Bay most plastic waste Nestlé could Unilever and Procter & Gamble are assigned. Several million tons of plastic waste drift in so-called garbage whirlpools in the North Pacific and North Atlantic . Every year this garbage kills hundreds of thousands of higher sea animals. Small pieces of plastic and microplastics end up in the food chain of marine animals and cause animals to starve to death or suffer internal injuries on a full stomach. Animals often mistake plastic parts for their food and swallow them. Larger plastic parts such as tarpaulins, defective fishing nets or ropes injure marine animals. Plastic tarpaulins cover coral sticks, sponges or mussel banks and thus prevent their colonization. According to a study by UNEP , the vortex in the Pacific contains up to 18,000 plastic parts on every square kilometer of sea surface. There are six kilograms of plastic for every kilogram of plankton. The sizes of the vortices can hardly be specified because they are not sharply delimited. In Switzerland, around 14,000 tons of plastic end up in the soil and water every year . Most of it ends up in the soil.
So far there are no specific studies on the effects of microplastics on humans. Studies on polymers that are used as carriers for drugs show that particles in the nanometer range are absorbed into the bloodstream, but are also excreted again.
Persistence
For a long time, plastics were considered to be non-biodegradable; only recently have some organisms been found that can break down plastics (see section Biodegradation of conventional plastics ). Chemical and physical processes take a very long time to break down plastics, since disintegration times of several hundred years have been calculated on such paths, plastics are also referred to as persistent . One possibility for inorganic degradation is the action of UV radiation (sunlight), in which case the plastic chains "break" piece by piece, which manifests itself macroscopically in yellowing and / or becoming brittle .
Basically, microorganisms can only process plastics through extracellular enzymes , which break the material down into smaller components that can then be absorbed by the cell. However, the enzymes are too large to effectively penetrate the rotting material, so that this process can only take place as surface erosion .
If toxic intermediate stages arise during the degradation through biochemical processes, these can accumulate in nature. Additives in plastics such as plasticizers , dyes or flame retardants pose an additional risk . In particular, volatile organic compounds should be mentioned as sources of pollution .
Waste management
Of the approximately 6.3 billion tons of plastic that became waste by 2015 , approximately 9% was recycled and 12% incinerated. About 79% of the plastics were dumped in landfills or were released into the environment, where they now accumulate. In Switzerland , around 90% of plastic waste is used to generate energy.
Plastic recycling
In 2012, the global recycling rate for plastic waste was only around 3% with an annual global annual production of plastics of around 280 million tons. In 2018, an article in the NZZ mentioned a figure of 8%. Much of the plastic waste produced is instead dumped in landfills or incinerated, and an estimated 20 million tonnes of non-recycled plastic end up in the oceans, where it is a huge environmental problem. In contrast, plastics are no longer deposited in Germany and Switzerland. In the EU, this target is to be achieved by 2020. In the Federal Republic of Germany, the recycling rate was 45% in 2010, making Germany a pioneer in European comparison. The remaining 55% is thermally recycled ( waste incineration ). The plastics industry has started a campaign Zero Plastics to Landfill by 2020 to support this project . There are now also industrial companies that have specialized in recycling plastic.
Basically, there are three ways of recycling:
Material recycling
Thermoplastics, once formed into a workpiece, can be melted down again and shaped into a new product. In many processes, however, the sequence of heat treatments leads to a progressive loss of quality of the material (downcycling) . The biggest problem with renewed material recycling, however, is the separation of the individual plastics. If different polymers are mixed in one material, this leads to a severe loss of quality and significantly poorer mechanical properties. To facilitate separation, the recycling code was introduced in 1988 . The recycling of non-sorted waste, such as household waste, is still difficult. The current separation processes are very labor-intensive and require a large amount of water and energy, so that both a cost-benefit calculation and the ecological balance are negative.
Material recycling is therefore currently used almost exclusively where large quantities of a single-origin material are available. For example, foam polystyrene packaging is collected in Germany that can be reused as a soil improver in agriculture or in the production of foam polystyrene concrete or bricks. The recycling rate for expanded polystyrene in 2000 was around 70%. There is also a take-back system for PVC, mainly floor coverings, roofing membranes, window profiles and PVC pipes are collected. Further areas of application for material recycling are, for example, in the recycling of vehicles or beverage bottles, or in countries of the second or third world, where the collection of single-type plastic waste contributes to income. In this way, packaging or products such as window profiles, pipes, flower and beverage boxes, new foils, window frames or watering cans are made from the secondary raw materials.
Recycling of raw materials
By means of pyrolysis , plastics can be broken down again into the respective monomers or other petrochemically usable substances such as methanol or synthesis gas . However, the availability of pure material is also a prerequisite for obtaining the monomers. Examples are the Hamburg process , which is currently operated by BP and is used both for the production of monomers and petrochemical raw materials, and the degradative extrusion process developed by Walter Michaeli and others, which is able to convert mixed plastic waste into gases that can be used as raw materials To convert waxes and oils. Naturally, these processes are primarily used for the recycling of mixed plastics, which can only be separated with great effort.
Energy recovery
When recovering energy, the plastics are used to generate energy. This happens almost exclusively through incineration . Areas of application are mainly blast furnaces , cement works , power plants etc. The high temperatures prevailing there ensure complete and low-emission combustion. The calorific value of plastics roughly corresponds to that of hard coal .
Plastic tax and incentive taxes
In order to reduce the quantity, spread in the environment and influence production by means of price incentives, a tax with a steering effect and the reduction of tax advantages over fuels are being considered and discussed controversially.
Dismantling
Conventional plastics were previously considered to be non-biodegradable. Without biodegradation, plastics only decompose very slowly through chemical and physical processes (see section Persistence ). More recently, some organisms have been found that can also break down conventional plastics.
Biodegradable plastics, mostly polyester, have been known for a long time .
Biodegradation of conventional plastics
It is known that two insects, the dried fruit moth Plodia interpunctuella and the large wax moth Galleria mellonella , can break down polyethylene with the help of intestinal bacteria.
The moth larva Galleria mellonella can perforate polyethylene films within a few hours. The exact mechanism is still unknown, presumably there is a connection with the ability of the moth larvae to digest wax from honeycombs (wax and polyethylene are chemically similar ; CH 2 -CH 2 bonds play an important role in both ). In 2016 the bacterium Ideonella sakaiensis was discovered, which is able to feed on PET waste. However, it took six weeks for a thin plastic film to break down. Since it breaks down PET into its raw materials terephthalic acid and glycol, it could in principle be used for recycling.
Likewise mealworm (Tenebrio molitor) are fed exclusively with polystyrene, which is still unknown at flour beetles of the degradation process. Since insect larvae process plastic into fine particles with their chewing tools, bacterial enzymes can break them down more quickly.
In 2017 it was shown in a publication that Tübingen watering can mold ( Aspergillus tubingensis ) can break down polyester urethanes . A plastic film was able to be completely broken down within two months.
Biodegradable plastics

Since around 1990 intensive research has been carried out on compostable, disposable plastics. Defined test of compostability of plastics since 1998 under the DIN standard V 54,900th order for a plastic biodegradable is, he must attack sites for the enzymes provide the microorganisms to use it for their own metabolism. These enzymes turn the long polymer chains into more manageable water-soluble fragments. For this purpose, naturally occurring polymers ( biopolymers ) or units can be integrated into synthetically produced chains such as sugar, succinic acid or lactic acid . The presence of heteroatoms such as nitrogen or oxygen in the plastic is crucial. Most of the around 30 marketable, biodegradable plastics known to date are polyesters, polyamides, polyester urethanes and polysaccharides. The problem with synthetically produced polyesters and amides is that the very properties that make up the impact and tensile strength of the materials (intramolecular hydrogen bonds in amides, aromatic components in polyesters) prevent their use by nature. An improvement in biodegradability almost always means a deterioration in the material properties. World production of biodegradable plastics was 300,000 tons in 2007 (compared to 240 million tons of standard plastic).
Polysaccharides
Polysaccharides ( starch , cellulose ) are used by nature as energy stores and structural substances. Countless simple sugars (such as glucose or fructose ) form long chains and thus represent naturally occurring polymers which, as such, can also be broken down by nature. They are cheap and available in large quantities, but they have a serious disadvantage: they cannot be processed into films, molded parts or fibers by melting; i.e., they are not thermoplastically moldable. However, thermoplastic moldability is precisely one of the great advantages of plastics. An esterification Although the free OH groups of the sugar improves the material properties, but also reduces their ability to biodegradability. If polysaccharides are used as a material, a compromise between material properties and biodegradability is necessary.
Polyhydroxybutyric acid (PHB)
Polyhydroxybutyric acid is a naturally occurring polymer that is formed by certain microorganisms to store energy. Through fermentation , these can be stimulated to enrich the polymer up to 90% of their own mass. As a biopolymer, it is biodegradable and exhibits material properties that are similar to those of polyesters. There are currently efforts to produce PHB in genetically modified plants ("plastic potatoes") .
- Possible areas of application
- Agriculture: rotting mulch films , plant pots
- Waste disposal: Disposal of particularly dirty, poorly recyclable garbage, such as food packaging, diapers, etc.
- Landscape maintenance : reducing littering
- Fisheries: Lost fishing nets pose a latent threat to larger marine life
- Artificial fertilizer: as a coating substance for fertilizer, so that it can act more slowly and in a more dosed manner (controlled release) .
Economic share of plastics
Overview
Around 380 million tons of plastic are currently used worldwide each year (as of 2017). On average, the production of plastics has grown by around 8.4% per year since 1950, which is 2.5 times as fast as the average gross domestic product .
There are various reasons for this (see also the Properties chapter ): First of all, crude oil is easily accessible as a raw material source; The share in the global oil consumption of plastics is only 4%. The weight of plastic is very low compared to iron and ceramic materials. The processing of plastics (and especially thermoplastics) is possible at low temperatures and therefore inexpensive. Finally, thanks to their special properties, plastics can also be used as functional materials (see chapter Special plastics ) for applications for which no other material would otherwise be suitable and which are in part only made possible by plastics.
In economic statistics , chemical fibers and synthetic resins in paints and adhesives are often shown separately from other plastics.
Production statistics of plastics
| year | World production ( jato ) |
|---|---|
| 1930 | 10,000 |
| 1949 | 1,000,000 |
| 1965 | 15,000,000 |
| 1976 | 50,000,000 |
| 2003 | 200,000,000 |
| 2008 | 280,000,000 |
| 2017 | 380,000,000 |
Production of plastics in Germany 2007
| Substance class | Annual production in tons | Sales in million euros | Average basic price in euros per ton |
|---|---|---|---|
| Polyethylene (d> 0.94) | 1,786,268 | 1,275 | 713.78 |
| Ethylene polyvinyl acetate | 31,424 | 43.5 | 1384.29 |
| Expandable polystyrene | 475,606 | 515 | 1082.83 |
| Different polystyrene | 426.272 | 145.5 | 341.33 |
| Uniform polyvinyl chloride | 1,564,029 | 1.101 | 703.95 |
| Plasticized polyvinyl chloride | 113.212 | 148.4 | 1310.82 |
| Vinyl chloride-vinyl acetate copolymers | 437,527 | 356.5 | 814.81 |
| Polyacetals | 140.244 | 333 | 2374.43 |
| Polyether alcohols | 566.463 | 562.7 | 993.36 |
| Epoxy resins (molding compounds) | 99,320 | 55.2 | 555.78 |
| Epoxy resins (adhesives) | 22.406 | 40.3 | 1798.63 |
| Epoxy resins (other) | 179,426 | 343.2 | 1912.77 |
| Polycarbonates | 326.279 | ||
| Alkyd resins | 255.622 | 208.4 | 815.27 |
| Polyethylene terephthalate | 534.093 | 615.1 | 1151.67 |
| Other polyester | 489,338 | 583 | 1191.41 |
| Polypropylene | 1,928,257 | 1,429 | 741.08 |
| Polypropylene copolymers | 493,962 | 298 | 603.29 |
| Vinyl acetate copolymers, aqueous (adhesives) | 227.115 | 132.2 | 582.08 |
| Acrylic polymers | 201,325 | 562.7 | 2794.98 |
| Acrylic polymers (molding compounds) | 600.940 | 801.9 | 1334.41 |
| Other acrylic polymers | 952.756 | 650.5 | 682.76 |
| Polyamides (molding compounds) | 587.024 | 1,296 | 2207.75 |
| Different polyamide | 610,896 | 732.6 | 1199.22 |
| Urea resins | 1,013,900 | 358 | 353.09 |
| Melamine resins | 355.616 | 338.9 | 952.99 |
| Other amino resins, polyurethanes | 493,962 | 298 | 603.29 |
| Other phenolic resins | 256604 | 296.5 | 1155.48 |
| Other polyurethanes | 1,065,888 | 1,666 | 1563.02 |
| Other silicones | 427.141 | 1,570 | 3675.60 |
| Cellulose ethers and derivatives | 188,348 | 544 | 2888.27 |
| Synthetic rubber, latex | 728.442 | 562.1 | 771.65 |
Health hazards
Polymers (main components of plastics) themselves are considered unreactive (inert) under physicochemical conditions of the body and cannot be absorbed by the cells of living organisms. Polymers are therefore probably harmless, but there are still many unanswered questions.
However, the possible dangers of microplastics are being investigated . Partially added additives can also pose a risk. Additives can escape (exudation) on the surface of the material, such as floor coverings . For this reason, particularly strict requirements regarding the use of additives apply to food packaging, plastics in medicine and similar applications. The plastics used in such areas require approval, for example by the FDA .
In this context, soft PVC in particular has been criticized in the past, as particularly large amounts of plasticizers are added to this plastic. It has therefore not been approved as packaging for food for a long time. The manufacture and sale of toys for children up to the age of three made of material that contains phthalate plasticizers (primarily DEHP ) is also prohibited in the European Union . However, to this day, toys made from soft PVC are mainly sold in the Far East.
The floor coverings, drinking bottles, food packaging, cosmetic containers, baby products, plastic toys, etc. manufactured and sold in the EU often also contain additives that are harmful to health. The endocrinological society Endocrine Society, the European Society of Endocrinology, the European Society for Pediatric Endocrinology, the German Society for Endocrinology , the National Institute of Environmental Health Siences, UNEP and the World Health Organization (WHO) consider it to be proven that plasticizers and Other plastic additives in the human body act as endocrine disruptors even in the smallest quantities and thus have a harmful influence on the endocrine system. According to this, numerous additives (including phthalates, parabens and phenols) are causally involved in the development of breast and prostate cancer, infertility, diabetes mellitus, cardiovascular diseases, thyroid diseases, childhood developmental disorders as well as neurological, neurodegenerative and mental diseases in humans.
Numerous medical societies from different countries criticize that the current limit values are inadequate and that much more regulatory efforts are necessary to protect consumers from the harmful effects of plastics. Although scientists have been warning of the dangers of endocrine disruptors for over 25 years, little policy has been taken to reduce consumer exposure to endocrine disruptors.
It is also criticized that the plastics producing industry has too great an influence on the approval, assessment and legislative process and that it tries to unilaterally influence public opinion and deny the scientific consensus on the dangerousness of plastics through targeted disinformation of the public and the infiltration of scientific journals . Methods similar to those used to postpone the regulation of asbestos and tobacco smoke in the twentieth century are used.
The largest and oldest endocrinological society in the world, the Endocrine Society, explicitly contradicts the assertions of manufacturers and state risk assessment institutes that there is no health risk if the current limit values are observed, and points out that there is no limit below which there is no harm to health could be assumed. She therefore recommends, among other things, to avoid serious health consequences:
- the avoidance of industrially produced and canned foods
- avoiding plastic storage media (especially those marked with the recycling code 3, 6 and 7). No heating in plastic products (e.g. in the microwave)
- avoiding the use of plastic bottles
- avoiding plastic toys
- avoiding products that contain endocrine disruptors (phthalates, bisphenol A, parabens)
- the use of cosmetics without synthetic fragrances
- avoiding contact with thermal paper, as is often used for receipts or the like.
- the diet through organic food, since no synthetic pesticides may be used in their production
literature
Books
- Erwin Baur et al. (Ed.): Saechtling plastic pocket book. 31st edition. Carl Hanser Verlag, Munich 2013, ISBN 978-3-446-43442-4 .
- Otto black, Friedrich-Wolfhard Ebeling: plastics customer. 10th edition. Vogel Business Media, Würzburg 2016, ISBN 978-3-8343-3366-7 .
- Gottfried W. Ehrenstein : Polymer materials. 3. Edition. Carl Hanser Verlag, Munich 2011, ISBN 978-3-446-42283-4 .
- Peter Elsner et al. (Ed.): Domininghaus - Properties and Applications. 8th edition. Springer, Berlin / Heidelberg 2012, ISBN 978-3-642-16172-8 .
- Wolfgang Kaiser : plastics chemistry for engineers. 4th edition. Carl Hanser Verlag, Munich 2016, ISBN 978-3-446-44638-0 .
- Christian Bonten: Plastics Technology - Introduction and Basics. Carl Hanser Verlag, Munich 2016, ISBN 978-3-446-45223-7 .
- Georg Menges u. a .: Materials science, plastics. 6th edition. Carl Hanser Verlag, Munich 2011, ISBN 978-3-446-42762-4 .
- Reinhard Gächter, Helmut Müller (eds.): Plastic additives. Stabilizers, auxiliaries, plasticizers, fillers, reinforcing agents, colorants for thermoplastics. 3rd edition. Carl Hanser Verlag, Munich / Vienna 1990, ISBN 3-446-15627-5 .
- Jürgen Dispan: Plastics processing in Germany. Industry report 2013. (= IMU Information Service No. 4–2013 ). Stuttgart 2013. Link to the industry study
Magazines and articles
- Plastic magazine . The key figure magazine for the plastics and rubber industry. Hoppenstedt, Darmstadt from 1995 ISSN 0941-8520
- Plast processors (PV). international trade journal for processing, design and application of plastics. Hüthig, Heidelberg 1.1950, Apr.ff. ISSN 0032-1338
- Plastics, synthetics . Trade journal for the manufacture, processing and application of plastics and new materials. Vogt-Schild, Solothurn 23.1992,6ff. ISSN 1021-0601
- Plastics (KU). Materials, processing, application . Organ of German plastics trade associations. Trade journal for plastics technology. Hanser, Munich 1.1911ff. ISSN 0023-5563
- PlasticXtra . Trade journal for the plastics and rubber industry. Sigwerb, Zug 1.2010ff. ISSN 1664-3933
- Harald Cherdron: Modern Aspects of Plastics . In: Chemistry in Our Time . tape 9 , no. 1 , February 1975, p. 25-32 , doi : 10.1002 / ciuz.19750090105 .
- Karlheinz Hillermeier and Albrecht Hille: Polyester fiber reinforcement of thermosetting molding compounds, BMFT research report, T83–155 (1983), ISSN 0340-7608
- Klaus G. Kohlpp: Growth through the ages - development history of plastics . In: plastics . 5/2005, pp. 22-32 (2005).
- Klaus Möbius: Plastics all over the world. In: Chemiker-Zeitung . - Chemische Apparatur 83 (20) (1959), pp. 693-699, ISSN 0009-2894
- Hans Priess: To rename the plastics in "Polyplasts". In: Chemiker-Zeitung. 74 (21) (1950), pp. 265 ff., ISSN 0009-2894
- Dietrich Braun: The long road to the macromolecule - polymer research before Hermann Staudinger . In: Chemistry in Our Time . tape 46 , no. 5 , 2012, p. 310-319 , doi : 10.1002 / ciuz.201200566 .
- Andreas Kalberer, Delphine Kawecki-Wenger, Thomas Bucheli: Plastic in agriculture: the state of knowledge and recommendations for action for agricultural research, practice, industry and authorities . In: Agroscope Science . No. 89 , 2019 ( admin.ch [PDF; 1.8 MB ]).
Movie
- 2009: Plastic Planet . Critical documentary on the use and dissemination of plastics.
Institutes
- Fraunhofer Institute for Applied Polymer Research , (IAP) Potsdam-Golm
- Lüdenscheid plastics institute
- Institute for Plastics Processing (IKV), Aachen
- Institute for Technical and Macromolecular Chemistry (ITMC) Aachen
- Macromolecular Chemistry I , University of Bayreuth
- Institute for Polymers , at the ETH Zurich
- Leibniz Institute for Polymer Research Dresden V. Dresden
- Chair for plastics technology , Erlangen
- Institute for Technical and Macromolecular Chemistry , University of Hamburg
- Institute for Plastics Technology (IKT), Stuttgart
- Institute for Applied Macromolecular Chemistry , (IAMC) Stuttgart
- Süddeutsches Kunststoff-Zentrum (SKZ), research institute and training center for plastics technology, Würzburg
- Institute for Polymer Materials and Plastics Technology, (PuK) Clausthal-Zellerfeld
- Department for plastics technology , at the Montanuniversität Leoben
- Chair for plastics technology , Ilmenau
Associations
- AVK - Industrial Association for Reinforced Plastics e. V. (AVK) , Frankfurt a. M.
- GKV Gesamtverband Kunststoffverarbeitende Industrie eV (GKV), Berlin
- Industrievereinigung Kunststoffverpackungen eV (IK), Bad Homburg
- Fachverband Schaumkunststoffe und Polyurethane eV (FSK), Frankfurt a. M.
Web links
- boell.de : Plastic atlas: Data and facts about a world full of plastic 2019 (PDF)
- chemie.fu-berlin.de: plastic table
- act.greenpeace.de: Ways out of the plastic crisis
- Company magazine: Plastic is the future, boy . ( Memento from January 19, 2008 in the Internet Archive ) (About image change and importance of plastic)
- Association of European plastics manufacturers : plasticseurope.org
- visualcapitalist.com, July 3, 2019, Iman Ghosh: Mapping the Flow of the World's Plastic Waste (English; "Map of the global plastic waste path")
Individual evidence
- ↑ On the different language usage in East and West Germany see: Jürgen Eichhoff: On some geographical differences in word usage in the German language that emerged in the 20th century. In: Language and Customs. Festschrift Martin, 1980, pp. 163–166.
- ↑ a b c d Roland Geyer et al .: Production, use, and fate of all plastics ever made . In: Science Advances . tape 3 , 2017, p. e1700782 , doi : 10.1126 / sciadv.1700782 .
- ^ Deutsches Kunststoff-Museum: Recipe for making artificial horn .
- ↑ a b c d e f g h Handbook of experimental chemistry, secondary level II, volume 12, plastics, recycling, everyday chemistry , p. 21, Aulis & Deubner-Verlag.
- ↑ E. Simon: About the liquid Storax (Styryx liquidus), in Liebigs Annalen der Chemie Volume 31 (1839), p. 265, doi: 10.1002 / jlac.18390310306 .
- ↑ Patent DE281687 : Process for the production of technically valuable products from organic vinyl esters. Registered on July 4, 1913 , published on January 18, 1915 , applicant: Chemische Fabrik Griesheim-Elektron , inventor: Fritz Klatte.
- ↑ Coroplast company history on coroplast.de, accessed on August 26, 2012.
- ^ Hermann Staudinger: About polymerisation. In: Reports of the German Chemical Society 53, 1920, p. 1073. doi: 10.1002 / cber.19200530627 .
- ^ Hermann Staudinger: The structure of the rubber. VI. In: Reports of the German Chemical Society. Treatises, Section B 57B, 1924, pp. 1203-1208.
- ↑ Hermann Staudinger: The chemistry of high molecular weight organic substances in the sense of Kekulesches structural theory. In: Reports of the German Chemical Society. 59, 1926, pp. 3019-3043.
- ^ Hermann Staudinger, K. Frey, W. Starck: Compounds of high molecular weight IX. Polyvinyl acetate and polyvinyl alcohol. In: Reports of the German Chemical Society. Treatises, Section B 60B, 1927, pp. 1782-1792.
- ↑ Patent DE961537 : Process for the production of aluminum trialkyls and aluminum alkyl hydrides . Applied on February 2, 1954 , published on April 11, 1957 , applicant: K. Ziegler, inventor: K. Ziegler, H.-G. Gellert.
- ^ Karl Ziegler, Hans Georg Gellert, Herbert Lehmkuhl, Werner Pfohl, Kurt Zosel: Organometallic compounds. XXVI. Trialkylaluminum and dialkylaluminum hydride from olefins, hydrogen, and aluminum. In: Ann. 629, 1960, pp. 1-13.
- ↑ Patent US3257332 : Polymerization of ethylene. Registered on November 15, 1954 , published on June 21, 1966 , inventors: Karl Ziegler, Heinz Breil, Erhard Holzkamp, Heinz Martin.
- ↑ Patent US2781410 : Polymerization of ethylene in the presence of an aluminum trialkyl catalyst.
- ↑ Giulio Natta, I. Pasquon, A. Zambelli: Stereo Specific catalysts for the head-to-tail polymerization of propylene to a crystalline polymer syndiotacfic. In: Journal of the American Chemical Society . 84, 1962, pp. 1488-1490.
- ^ A b c Gerhard W. Becker, Dietrich Braun, Bodo Carlowitz: Die Kunststoffe. Chemistry, physics, technology . 1st edition. Hanser, 1990, ISBN 3-446-14416-1 , pp. 16-17 ( limited preview in Google Book search).
- ↑ a b c Hans-Georg Elias: Makromoleküle, Volume 4: Applications of polymers . 6th edition. Wiley-VCH, Weinheim 2003, ISBN 3-527-29962-9 , pp. 290–294 ( limited preview in Google Book search).
- ↑ Entry on elastomers. In: Römpp Online . Georg Thieme Verlag, accessed on 2014-12-09.
- ↑ Wolfgang Kaiser : Kunststoffchemie für Ingenieure: From synthesis to application . 2nd Edition. Carl Hanser, 2007, ISBN 978-3-446-41325-2 , pp. 119 ( limited preview in Google Book search).
- ↑ Density of ceramic materials at keramverband.de. Retrieved January 24, 2014 .
- ↑ Wolfgang Seidel: Materials technology, materials - properties - testing - application. Hanser Verlag, Munich 2008, 7th edition, ISBN 978-3-446-40789-3 , p. 243.
- ↑ a b c J.MG Cowie: Chemistry and Physics of Synthetic Polymers . Vieweg, 2nd Ed., 1997, ISBN 3-528-06616-4 .
- ^ Friedrich E. Neumann: α-Methylstyrene block copolymers. In: German Offenlegungsschrift 1970, p. 13.
- ↑ M. Szwarc: Living polymers. Their discovery, characterization, and properties. In: Journal of Polymer Science , Part A: Polymer Chemistry, 36 (1), ix 1998.
- ↑ H.-G. Elias: Volume 3: Industrial Polymers and Syntheses . Wiley-VCH, edition: 6th, completely revised. Edition. 2001, ISBN 3-527-29961-0 .
- ↑ Intermediate amines are formed which, however, react immediately with isocyanate to form water-insoluble ureas.
- ^ A b c W. Hohenberger: Additives. Trends and perspectives. In: plastics. 92 (5), 2002, pp. 86-91.
- ↑ DINCH as a DEHP replacement? In: Allum - Allergy, Environment and Health . ( allum.de [accessed on January 18, 2018]).
- ^ A b c W. Keim, A. Behr, G. Schmitt: Fundamentals of industrial chemistry . Sauerländer Verlag, 1986.
- ↑ a b c d e f H.-G. Elias: Polymers From Monomers and Macromolecules to Materials . Hüthig & Wepf, Midland, 1996, ISBN 3-8252-8107-8 .
- ↑ Craig S. Barrow, Helen Lucia, Yves C. Alarie: A comparison of the acute inhalation toxicity of hydrogen chloride versus the thermal decomposition products of polyvinylchloride. In: Journal of Combustion Toxicology . 6 (Feb.), 1979, pp. 3-12.
- ↑ F. de Bie: The crucial question in fire protection, flame-retardant thermoplastics - strategy and development. In: plastics. 92 (2), 2002, pp. 70-73.
- ^ Matthias C. Hund, Norbert Grünewald: Do it yourself: The Latest Additives and Masterbatches . In: Kunststoffe international . tape 93 , no. 7 , 2003, p. 38-39 ( online ).
- ^ Martin Bonnet: Plastics in engineering applications. ISBN 978-3-834-89303-1 , p. 166 ( limited preview in Google book search).
- ↑ Plastics industry . PlasticsEurope Germany e. V. ( Memento of March 11, 2007 in the Internet Archive ), accessed on October 4, 2007.
- ^ A b c W. Kaiser: Kunststoffchemie für Ingenieure . Hanser, Munich 2006, ISBN 3-446-22069-0 .
- ↑ https://de.statista.com/statistik/daten/studie/167099/umfrage/weltproduktion-von-kunststoff-seit-1950/ (accessed on June 13, 2019)
- ↑ Manas Chanda, Salil K. Roy: Industrial Polymers, Specialty Polymers, and Their Applications (= Plastics Engineering ). CRC Press, 2008, ISBN 978-1-4200-8058-2 , pp. 2 ( limited preview in Google Book search).
- ↑ Nitash P. Balsara, Daniel T. Hallinan: Polymer Electrolytes . In: Annual Review of Materials Research . doi : 10.1146 / annurev-matsci-071312-121705 .
- ^ Norio Ise, Ikuo S. Sogami: Structure Formation in Solution: Ionic Polymers and Colloidal Particles . Springer, Berlin / Heidelberg 2005, ISBN 3-540-27715-3 , pp. 1 .
- ↑ LC Sawyer, RT Chen, MG Jamieson, IH Musselman, PE Russell: The fibrillar hierarchy in liquid crystalline polymers. In: Journal of Materials Science . 28 (1), 1993, pp. 225-238.
- ↑ Charles E. Carraher Jr .: polymer processing. Spinning and fiber production. In: Polymer News . 27 (3), 2002, pp. 91-94.
- ↑ JM Schmitt, M. Beeres: The medical technology industry: a competence center for health. In: Medical device technology . 17 (3), 2006, pp. 52/53.
- ↑ a b c d e A. C. Gore, VA Chappell, SE Fenton, JA Flaws, A. Nadal: EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals . In: Endocrine Reviews . tape 36 , no. 6 , December 2015, ISSN 0163-769X , p. E1 – E150 , doi : 10.1210 / er.2015-1010 , PMID 26544531 , PMC 4702494 (free full text).
- ↑ a b c d WHO | State of the science of endocrine disrupting chemicals - 2012. Retrieved March 11, 2019 .
- ↑ Introduction to Endocrine-Disrupting Chemicals | Endocrine Society. Retrieved March 11, 2019 .
- ^ Bullfrog Films: Addicted to Plastic
- ↑ Chris Jordan, Message from the gyre on Vimeo (trailer)
- ↑ Badische-zeitung.de , April 13, 2015, Claudia Füßler: Plastic traces in food are omnipresent
- ↑ Annett Stein (dpa): Plastic waste poisons key species in the North Sea . Die Welt, December 7, 2013.
- ↑ Raveender Vannela: Are We “Digging Our Own Grave” Under the Oceans? In: Environmental Science & Technology . tape 46 , no. 15 , August 7, 2012, p. 7932-7933 , doi : 10.1021 / es302584e ( PDF ). PDF ( Memento from February 2, 2015 in the Internet Archive )
- ↑ Mark Gold et al. a. (Emmet Center on Climate Change and the Environment): Stemming the Tide of Plastic Marine Litter: A Global Action Agenda Pritzker Environmental Law and Policy Briefs ; Policy Brief No. October 5 , 2013.
- ↑ Julien Boucher, Damien Friot: Primary Micro Plastics in the Oceans. (PDF) IUCN, accessed August 21, 2017 .
- ↑ Yves Zenger: Coca-Cola, Pepsi and Nestlé are flooding the world with plastic In: greenpeace.ch, October 9, 2018, accessed on October 10, 2018.
- ↑ Greenpeace : Nestlé, Unilever, P&G among worst offenders for plastic pollution in Philippines in beach audit In: greenpeace.org, September 22, 2017, accessed October 10, 2018.
- ↑ Our oceans are sinking in plastic waste WWF Germany, accessed on July 3, 2015.
- ^ Charles Moore: Out in the Pacific, Plastic is Getting Drastic (The World's Largest Landfill is in the Middle of the Ocean) (PDF; 26 kB) 2002.
- ↑ response.restoration.noaa.gov
- ^ Federal Office for the Environment: Plastics in the Environment. In: admin.ch. Retrieved May 16, 2020 .
- ↑ Federal Office for the Environment: Plastics in the Environment: Soils. (PDF; 97.3 kB) In: admin.ch. Retrieved May 16, 2020 .
- ↑ Nadja Podbregar: How much plastic gets into our environment? In: Wissenschaft.de . July 12, 2019, accessed on July 15, 2019 : "The soils contain 40 times more plastic than the water."
- ↑ Fraunhofer Institute (ed.): Plastics in the environment: micro- and macroplastics . Oberhausen June 2018, p. 31 ( fraunhofer.de [PDF]).
- ↑ a b c d e f g h i j k l R.-J. Müller: Biology in our time , 30, 2000, p. 218.
- ↑ spiegel.de , February 2, 2008, Samiha Shafy: Das Müll-Karussell , accessed on February 7, 2008.
- ↑ What to do with Europe's garbage now that China no longer wants it? In: tagesanzeiger.ch . January 4, 2018, accessed January 11, 2018.
- ↑ Plastic waste flows take new paths , NZZ, June 22, 2018, p. 24.
- ↑ Holding back the tide in a sea of plastic ( Memento from February 2, 2015 in the Internet Archive ) European Biotechnology, Winter Edition, Vol. 13, 2014.
- ↑ In 2010, landfill continued to make up almost 40% of treated municipal waste in the EU27. Eurostat press office, STAT / 12/48, 27 March 2012.
- ↑ plasticseurope.org: IdentiPlast 2012: zero plastics to landfill by 2020 - how to reach the goal? ( Memento of the original from November 11, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. . Retrieved November 9, 2012.
- ↑ a b Brigitta Huckestein, Thomas Plesnivy: Possibilities and limits of plastics recycling . In: Chemistry in Our Time . tape 34 , no. 5 , October 2000, p. 276-286 , doi : 10.1002 / 1521-3781 (200010) 34: 5 <276 :: aid-ciuz276> 3.0.co; 2-q .
- ↑ W. Kaminsky, H. Sinn: Petrochemical processes for recycling plastics. Pyrolytic techniques. In: Recycling and Recovery of Plastics . 1996, pp. 434-443.
- ↑ P. Menges, W. Michaeli, V. Lackner: MONTHS FOR CHEMISTRY , 127 (8), 1996, p. 821.
- ↑ Environment Minister Schulze rejects plastic tax , Die Zeit , May 11, 2018, accessed on May 23, 2018.
- ↑ Paolo Bombelli, Christopher J. Howe, Federica Bertocchini: Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella . In: Current Biology . tape 27 , no. 8 , April 24, 2017, ISSN 0960-9822 , p. R292 – R293 , doi : 10.1016 / j.cub.2017.02.060 ( cell.com [accessed April 27, 2017]).
- ↑ Shosuke Yoshida, Kazumi Hiraga, Toshihiko Takehana, Ikuo Taniguchi, Hironao Yamaji: A bacterium that degrades and assimilates poly (ethylene terephthalate) . In: Science . tape 351 , no. 6278 , March 11, 2016, ISSN 0036-8075 , p. 1196-1199 , doi : 10.1126 / science.aad6359 , PMID 26965627 .
- ↑ A bacterium that eats plastic - science.ORF.at. Retrieved April 28, 2017 .
- ↑ Sehroon Khan, Sadia Nadir, Zia Ullah Shah, Aamer Ali Shah, Samantha C. Karunarathna, Jianchu Xu, Afsar Khan, Shahzad Munir, Fariha Hasan: Biodegradation of polyester polyurethane by Aspergillus tubingensis . In: Environmental Pollution . tape 225 , p. 469-480 , doi : 10.1016 / j.envpol.2017.03.012 .
- ^ Dietrich Braun: 150 years of plastics - success through research. Chemische Rundschau, October 2008, p. 78ff.
- ^ Y. Doi: Microbial Polyesters. VCH Publishers, New York 1990.
- ↑ Wolfgang Kaiser : Kunststoffchemie für Ingenieure: From synthesis to application . 2nd Edition. Carl Hanser, 2007, ISBN 978-3-446-41325-2 , pp. 4–5 ( limited preview in Google Book Search).
- ↑ Federal Statistical Office, Series 4, Series 3.1, 2007.
- ↑ Holger Sieg, Linda Böhmert, Alfonso lamps: Microplastics in food: Oral intake, toxicology and risk assessment . In: Federal Institute for Risk Assessment (Hrsg.): UMID - Environment + People Information Service . NO . 1/2019, January 2019 ( Umweltbundesamt.de [PDF]).
- ↑ P. Stolz, N. Weis, K. Schäffer, H. Kleemeyer, M. Toben: Plastics - Environmental and Health Hazards . Bremer Umweltinstitut e. V., Environmental Foundation WWF-Germany, Bremen 1995, ISBN 3-9803930-2-X .
- ↑ Endocrine Disrupting Chemicals (EDCs) | Hormone Health Network. Retrieved March 12, 2019 .
- ↑ PFAS - Per- and Polyfluoroalkyl Substances. Retrieved March 11, 2019 .
- ↑ Endocrine Disruptors Research. Retrieved March 11, 2019 .
- ↑ Stricter regulations needed to protect against harmful environmental hormones - www.endokrinologie.net. Retrieved March 11, 2019 .
- ↑ Endocrine experts united in disappointment with European Commission's proposed criteria on EDCs | Endocrine Society. Retrieved March 11, 2019 .
- ↑ Brett Aho: Disrupting regulation: understanding industry engagement on endocrine-disrupting chemicals . In: Science and Public Policy . tape 44 , no. 5 , October 1, 2017, ISSN 0302-3427 , p. 698-706 , doi : 10.1093 / scipol / scx004 ( oup.com [accessed March 11, 2019]).
- ↑ Barbara Casassus: Hormone disrupting chemicals: slow progress to regulation . In: BMJ . April 30, 2018, ISSN 0959-8138 , p. k1876 , doi : 10.1136 / bmj.k1876 ( bmj.com [accessed March 11, 2019]).
- ↑ EDCs Myth vs. Fact | Hormone Health Network. Retrieved March 11, 2019 .
- ↑ Endocrine Disrupting Chemicals (EDCs) | Hormone Health Network. Retrieved March 11, 2019 .