Peroxides

Peroxides , also spelled peroxides out of date , are a chemical group of substances that contain the peroxide anion O 2 2− or a peroxy group –O – O–. In contrast to the oxide ion , the most common anion of oxygen , have the oxygen atoms in the peroxide ion, the oxidation state -1.
The group of peroxidic compounds can initially be roughly divided into inorganic and organic peroxides, the inorganic often having a salt-like character , whereas the organic ones carry covalently bound peroxy groups. The oxygen-oxygen bond of peroxides is labile and tends to split homolytically with the formation of reactive radicals . For this reason, peroxides are only found in small amounts in nature, including in water and the earth's atmosphere and in small amounts in plant, animal and human organisms .
Peroxides are of industrial importance because of their bleaching effect on organic substances. They are added to detergents , among other things under the advertising name active oxygen , or used in hair dye products. Other areas of application include the chemical industry , in which peroxides are used for synthesis or occur as intermediate products, and medicine . With an annual production volume of over 2,000,000 tons, hydrogen peroxide is the economically most important peroxidic compound.
Often the peroxides are unstable and therefore cannot be stored and are sometimes dangerous substances. That is why they are often produced on site ( in situ ) and implemented immediately.
history
As probably the first synthetically produced peroxo compound, Alexander v. Humboldt in 1799 during attempts to break down the air with barium peroxide . It wasn't until 19 years later that Thénard realized that this salt could be used to produce a previously unknown compound that he called oxidized water - now known as hydrogen peroxide. In 1811 Thénard and Gay-Lussac first produced sodium peroxide . In sustained research efforts over the following decades, hydrogen peroxide and its salts were investigated. In the search for a use, the bleaching effect of the compound on natural dyes was recognized early on. However, industrial use initially failed because only weakly concentrated and contaminated barium peroxide solutions could be produced. The first industrial plant for the synthesis of hydrogen peroxide was built in Berlin in 1873 . Only after the discovery of the synthesis of hydrogen peroxide by the electrolysis of sulfuric acid could improved processes on an electrochemical basis be developed. The first factory to use this method was built in 1908 in Weißenstein in Carinthia . The anthraquinone process still used today was developed by IG Farben in Ludwigshafen during the 1930s . With more modern synthesis processes and the expansion of the field of application, the annual production of hydrogen peroxide rose sharply from 35,000 t in 1950 to 100,000 t in 1960 to 300,000 t in 1970. In 1998 there was a worldwide production capacity of 2,700,000 t per year.
Occurrence
In the environment
Peroxides are usually very reactive, which is why there are only few natural occurrences. In addition to hydrogen peroxide, this includes a few natural plant substances such as a peroxidic derivative of prostaglandin and ascaridol . Hydrogen peroxide occurs naturally in surface water, groundwater, and in the earth's atmosphere . The formation takes place here through the action of light or natural catalytically active substances from water . Sea water contains 0.5–14 μg / l, fresh water 1–30 μg / l, and air 0.1–1 ppb .
Two peroxide-containing minerals are known, studtite and metastudtite . These are uranyl peroxides with different amounts of crystal water in the structure. The unstable peroxide is created during the radiolysis of water by the alpha radiation of uranium . Apart from in natural uranium deposits, these compounds also form on the surface of radioactive waste and their stability could therefore be of importance for the final storage of uranium waste.
In biochemical processes
Hydrogen peroxide is also formed in human and animal organisms. It occurs as a short-lived product in biochemical processes and belongs to the cell toxins . The toxicity is based on changes in proteins , membrane lipids and DNA through oxidative reactions of the peroxide ions. The enzyme superoxide dismutase , which serves to remove superoxide ions , produces hydrogen peroxide in vivo by disproportionation . This is then rapidly broken down into oxygen and water by the enzyme catalase .
- Formation of hydrogen peroxide by superoxide dismutase (SOD).
- Breakdown of hydrogen peroxide by catalase (CAT)
Peroxisomes are cell organelles in eukaryotic cells that are used for the oxidative breakdown of fatty acids . During the breakdown, hydrogen peroxide is produced in them according to the following equation:
The resulting hydrogen peroxide is then converted in turn by catalase.
Another source of hydrogen peroxide is the breakdown of adenosine monophosphate . This is first converted into hypoxanthine in a sequence of biochemical reactions . This is then oxidatively catabolized first to xanthine and then to uric acid . This reaction is catalyzed by the enzyme xanthine oxidase and one equivalent of hydrogen peroxide is produced per formula conversion.
The breakdown of guanosine monophosphate also takes place via xanthine as an intermediate product, which is then converted into uric acid in the same way with the formation of hydrogen peroxide.
In the egg cells of sea urchins , hydrogen peroxide is produced for a short time after fertilization by a sperm. This is dissociated to OH radicals and serves as an initiator of a radical polymerization , which surrounds the egg cell with a sealing polymeric protective layer.
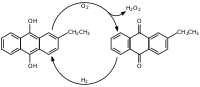
The bombardier beetles have an explosive device on their abdomen with which they can shoot caustic and foul-smelling bubbles at enemies for their own defense. To blow out a bubble, hydrogen peroxide and hydroquinone are mixed with one another shortly beforehand , which react violently with one another and lead to the launch of the chemical mixture.
Furthermore, hydrogen peroxide is a signaling molecule in the plant's defense against pathogens .
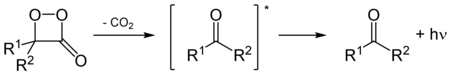
In fireflies , 1,2-dioxetanes are formed through the oxidation of luciferins . This reaction is catalyzed by luciferases . The resulting dioxetane is labile and spontaneously breaks down to carbon dioxide and an excited ketone . This relaxes by releasing a photon into the ground state , which causes this species to glow.
binding
The peroxide ion is made up of two oxygen atoms which are linked to one another via a single bond . This is in agreement with the MO diagram of the peroxide ion, which predicts a bond order of 1 due to the doubly occupied antibonding π * molecular orbitals . The bond length is 149 pm and is longer than that of the oxygen molecule ( triplet oxygen ( 3 O 2 ): 121 pm) due to the increasing population of antibonding orbitals. This is also expressed in the smaller force constant of the bond (2.8 N / cm, 3 O 2 : 11.4 N / cm) and the lower frequency of the molecular oscillation (770 cm −1 , 3 O 2 : 1555 cm −1 ) out.
In comparison to the other oxygen molecule ions ( hyperoxide ion : O 2 - and ozonide ion O 3 - ) and the oxygen molecule, the peroxide ion is the only one not a radical and not paramagnetic .
Due to the weak bond between the oxygen atoms, homolytic cleavage can easily be induced. Two radical fragments are formed, which in most cases have a high reactivity.
The cleavage can be triggered by temperature ( thermolysis ), light waves ( photolysis ) or chemical reactions .
Inorganic peroxides

The group of inorganic peroxides is divided into the classes of ionic peroxide salts and those of inorganic acid peroxides, which carry covalently bound peroxide units . While the peroxides of the alkali and alkaline earth metals are essentially of technical importance from the first class , the most prominent representatives of the covalent hydrogen peroxide are . In addition, a number of peroxides of mineral acids such as Caro's acid and percarbonic acid are important. In contrast to the purely ionic character of the alkali metal peroxides, the peroxides of the transition metals have a more covalent character, which is due to their higher electronegativity .
presentation
Synthesis of ionic peroxides
Alkali metal peroxides, with the exception of lithium peroxide , can be prepared directly by oxidizing the elements with oxygen under normal pressure .
- M = alkali metal.
Lithium peroxide, on the other hand, can be synthesized by reacting lithium hydroxide with hydrogen peroxide.
The barium peroxide historically used for the production of hydrogen peroxide can be obtained by oxidizing barium oxide at elevated temperature and pressure.
Synthesis of covalent peroxides
The most widely used method for the synthesis of hydrogen peroxide is the anthraquinone method . In this first used anthraquinone is catalyzed by palladium and hydrogenated with molecular hydrogen . In a second step, the resulting anthrahydroquinone is oxidized with molecular oxygen to release hydrogen peroxide with the reformation of anthraquinone. The gross reaction equation is thus:
The direct synthesis of hydrogen peroxide from the elements is currently not possible on an industrial scale, as it is only formed to a small extent. Many peroxidic mineral acids can be synthesized by anodic oxidation of the underlying acids. For example, peroxodisulfates and percarbonates are accessible in this way.
Historically, peroxodisulfuric acid was used to produce hydrogen peroxide in a process developed at the beginning of the 20th century. The peroxodisulfuric acid obtained by oxidation was converted into hydrogen peroxide and sulfuric acid by hydrolysis .
properties
Few reactions can generally be formulated for the reactions of peroxide salts. In the case of all peroxide salts, when mixed with an excess of dilute acids, hydrogen peroxide is released.
- Acid hydrolysis of sodium peroxide to form caustic soda and hydrogen peroxide.
The reaction with water, as well as the thermolysis of the salt, leads to the formation of nascent oxygen.
- Hydrolysis of sodium peroxide to form caustic soda and oxygen.
- Thermolysis of sodium peroxide to form sodium oxide and oxygen.
use
Peroxides are strong oxidizing agents and can be used to oxidize other compounds.
Alkali metal peroxides can be used to synthesize organic peroxides. One example is the reaction of sodium peroxide with benzoyl chloride to form dibenzoyl peroxide .
Many inorganic peroxides are used as bleaching agents in the textile industry and in the paper industry . Due to the increasing development of environmentally friendly bleaching processes, the use of peroxide-containing compounds has risen sharply and has largely displaced the older approach based on the bleaching effect of chlorine- containing compounds. The main areas of application in the household include use in detergents . In the past, perborates were used for this purpose , which were largely replaced by percarbonates due to the increasing boron concentrations in the environment. The use of peroxidic compounds in detergents is also reflected in their trade names. Thus the name of the detergent is Persil from the words Per borate and Sil icat together.
By reacting with carbon dioxide , oxygen can be liberated from some peroxidic salts with the formation of carbonate salts . This is used in oxygen generators, for example in breathing apparatus, submarines or space capsules . In order to generate oxygen, the unwanted carbon dioxide in the breath, which is released into the environment as a metabolic product, is converted and the required oxygen is released. In most cases, sodium peroxide is used for this purpose, but lithium peroxide in space capsules, since it has a lower molar mass and is able to generate a relatively larger volume of oxygen per unit weight.
- Reaction of sodium peroxide with carbon dioxide to form sodium carbonate and oxygen.
Barium peroxide was historically used to produce pure oxygen from air. The temperature-dependent equilibrium between barium oxide and barium peroxide was used for this purpose . By reacting barium oxide with air, barium peroxide was initially formed at 500 ° C. At temperatures above 700 ° C, this releases oxygen with regression of barium oxide.
Calcium peroxide CaO 2 is used as an oxygen- releasing aeration agent in soils and pond sludge , in the USA also as a food additive for loosening dough
Organic peroxides
The group of organic peroxides is essentially divided into two classes of compounds, the peroxycarboxylic acids and the organic peroxides or hydroperoxides . The first class is derived from the carboxylic acids , the second formally from the ethers or alcohols .
Endoperoxides are understood as meaning cyclic organic compounds with a peroxide group that connects two atoms of a ring with one another. The addition endo means that the peroxide bridge is inside the ring.
presentation
Synthesis of peroxides and hydroperoxides
Several routes are known for the synthesis of aliphatic peroxides. The reaction of dialkyl sulfates with alkaline hydrogen peroxide solution can be used for this purpose. The alkyl sulfate acts as a donor of the alkyl group and the sulfate ion reacts in the sense of a leaving group .
In contrast, cyclic peroxides can be obtained from alkylene disulfates under the same conditions. The four-membered dioxetanes can be prepared by [2 + 2] cycloaddition of oxygen onto alkenes .
The targeted synthesis of hydroperoxides can be carried out by radical oxidation of alkanes with oxygen. The primary radical formed by a radical initiator reacts with oxygen to form a hydroperoxyl radical. This is a less reactive radical that is able to abstract activated hydrogen atoms with high selectivity, releasing the hydroperoxide with the formation of a new radical. This selectivity is used on an industrial scale , for example, in Hock's phenol synthesis .
This reaction also takes place slowly, induced by atmospheric oxygen, in substances that can form stable radicals. Examples of this are the autoxidation of solvents from the group of ethers that are frequently used , such as, for example, diethyl ether , diisopropyl ether , tetrahydrofuran or 1,4-dioxane . This creates explosive ether hydroperoxides , which can cause severe explosions when heated or concentrated.
Peroxides can be formed in organisms by an ene reaction or Diels-Alder reaction of alkenes with oxygen. Unsaturated fatty acids for the ene reaction or unsaturated amino acids such as histidine for the Diels-Alder cyclization can serve as olefinic substrates . The rancidity of fats is also partly caused by the formation of peroxides. Oxidations with atmospheric oxygen such as the ene reaction or radical reactions take place and form not only peroxides but also alcohols, aldehydes and carboxylic acids . The corruption of a fat or oil can thus be determined based on the peroxide number , which indicates the amount of peroxides per kilogram of substance.
Synthesis of peroxycarboxylic acids
Most peroxycarboxylic acids are accessible through the reaction of hydrogen peroxide with the corresponding carboxylic acid :
- R = organic residue
Another synthetic route is the use of carboxylic acid chlorides instead of the free carboxylic acid. This route is mainly chosen for aromatic carboxylic acids and carried out in a basic medium in order to neutralize the hydrogen chloride formed .
Aromatic aldehydes also undergo auto-oxidation to peroxycarboxylic acids:
- Ar = aryl
However, these react with further aldehyde to form the carboxylic acid:
properties
Peroxycarboxylic acids are generally weaker acids than the carboxylic acids on which they are based . Like most peroxidic compounds, they tend to be explosive in high concentrations and at higher temperatures.
In the presence of oxidizable compounds, they can act as oxidizing agents.
use
Organic peracids can be used to synthesize epoxides . This is what happens in the Prileshayev reaction . The Baeyer-Villiger rearrangement for the synthesis of lactones from cyclic ketones represents a further field of application for organic peroxides. In both cases, electron-poor peroxycarboxylic acids are particularly suitable for carrying out the reaction. A frequently used peracid is meta -chloroperbenzoic acid ( m CPBA).
The hydroperoxide tert -butyl hydroperoxide is in the Sharpless epoxidation to enantioselective synthesis of epoxides, for which, among others, the 2001 Nobel Prize in Chemistry to Barry Sharpless awarded was as oxidant .
Peracetic acid is used as a disinfectant in the medical field and the food industry. There are also peroxide-containing solutions for cleaning contact lenses commercially available.
Dibenzoyl peroxide is used as a radical initiator in both laboratory and industrial applications . The weak peroxidic bond can easily be split homolytically and thus forms reactive benzoyl radicals . These can be used on an industrial scale for polymerization to plastics such as polyethylene .
The synthesis of the ε-caprolactam required for plastic production is of industrial importance . For this purpose, ε-caprolactone is formed by a Baeyer-Villiger rearrangement of cyclohexanone with peracetic acid , which is then converted to lactam with ammonia .
Industrial synthetic resins based on acrylic and / or methacrylic acid esters are without exception produced by free-radical polymerization with organic peroxides at elevated temperatures. The rate of polymerization can be adjusted by a suitable choice of temperature and type of peroxide.
Few peroxidic agents could be produced. They include, for example, artesunate and artemisinin . Their mode of action is based on the formation of radicals at desired locations in the organism.
Due to the explosive effect of many peroxides, initial peroxide-based explosives were also developed and used. The best known of these include acetone peroxide (APEX) and hexamethylene triperoxide diamine (HMTD).
proof
Many analytical methods are described in the literature for the qualitative and quantitative determination of peroxides. The iodine-starch reaction is a simple qualitative proof of peroxides . Any peroxides, hydroperoxides or peracids present oxidize the added potassium iodide to iodine , which forms deep blue inclusion complexes in the presence of starch. This method is also suitable for quantitative determination, but does not differentiate between the different types of peroxidic compounds. To distinguish between peroxides and peracids, the discoloration of an indigo solution can be examined, which occurs immediately in the presence of peracids. The blue coloration that occurs in the presence of peroxides with leuco methylene blue , on the other hand, is specific for peroxides. Starch iodine papers are commercially available which can be used as a quick indicator of the presence of peroxides.
The potentiometric titration with lithium aluminum hydride is suitable for the quantitative determination of hydroperoxides . One way to quantitatively determine the content of peracids and peroxides is volumetric titration with alcoholates such as sodium ethanolate .
Safety measures
Peroxides are unstable compounds and can show explosive behavior, especially in higher concentrations and at elevated temperatures. Furthermore, they are fire-promoting and can react with oxidizable substances (including cotton wool and cellulose) to develop fire. For this reason, a number of safety measures must be observed when working with peroxides:
- The decomposition of peroxides with the formation of radicals is favored by increased temperature and incidence of light. For this reason, peroxidic compounds should be stored in a cool place in opaque containers.
- Substances that tend to form peroxides through auto-oxidation, such as diethyl ether or tetrahydrofuran (THF), should be stored in dark or opaque bottles over sodium hydroxide .
- If the presence of peroxides is suspected, it should be checked for the presence of peroxides before heating or concentration of a reaction solution.
- Small amounts of peroxides that escape from storage or reaction vessels should be destroyed by adding reducing agents such as iron (II) sulfate . Only then can the contamination be safely absorbed with paper.
The safe industrial handling of peroxides requires extensive safety measures. Organic peroxides are produced industrially in cell structures ( explosion protection ). The equipment is usually located in concrete cells with foil windows to relieve pressure in the event of an explosion. Further safety measures are trenches filled with water in front of the production cells, which can be flooded with the reaction solution in the event of thermal runaway . After production and filling in small containers, peroxidic compounds must be quickly brought to refrigerated storage.
Detailed accident prevention regulations are anchored in the professional association regulation DGUV regulation 13.
Web links
- Information and list of substance classes that tend to form peroxide (PDF file; 59 kB)
Individual evidence
- ↑ Entry on peroxides . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04510 Version: 2.3.3.
- ↑ LW Gilbert (Ed.): The dripping liquid oxygen, or the oxygenated water , in: Ann. der Physik 1820 , p. 3, Leipzig ( limited preview in the Google book search).
- ^ A b c d H. Offermanns , G. Dittrich, N. Steiner: Hydrogen peroxide in environmental protection and synthesis , in: Chemistry in our time 2000 , 34 , 150–159.
- ↑ Karrie-Ann Hughes Kubatko, Katheryn B. Helean, Alexandra Navrotsky, Peter C. Burns: Stability of Peroxide-Containing Uranyl Minerals. In: Science. 302, 2003, pp. 1191-1193, doi: 10.1126 / science.1090259 .
- ^ G. Löffler, PE Petrides: Physiologische Chemie . 4th edition, p. 288, Springer, Berlin 1988 , ISBN 3-540-18163-6 .
- ^ G. Löffler, PE Petrides: Physiologische Chemie . 4th edition, pp. 321-322, Springer, Berlin 1988 , ISBN 3-540-18163-6 .
- ^ Lehninger: Biochemistry . 3rd edition, pp. 663-664, Springer, 2001 , ISBN 3-540-41813-X .
- ↑ a b Lehninger: Biochemistry . 3rd edition, p. 932, Springer, 2001 , ISBN 3-540-41813-X .
- ↑ M. Kröger: Chronik , in: Chemie in our time 1989 , 23 , 34–35; doi: 10.1002 / ciuz.19890230106 .
- ↑ H. Schildknecht , K. Holoubek: Die Bombardierkäfer und their Explosionschemie , in: Angew. Chem. 1961 , 73 , 1-7; doi: 10.1002 / anie.19610730102 .
- ↑ Werner Nachtigall, A. Wisser: Biologisches Design , Springer-Verlag Berlin, 1st edition, 2005 , ISBN 978-3-540-22789-2 ( limited preview in the Google book search).
- ↑ Helmholtz Institute for Biochemical Plant Pathology: How Plants Protect themselves ( Memento from April 3, 2008 in the Internet Archive ).
- ^ A. Gossauer: Structure and reactivity of biomolecules: An introduction to organic chemistry , 1st edition, pp. 306-307, Wiley-VCH / Helvetica Chimica Acta, 2006 , ISBN 978-3-906390-29-1 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , pp. 504-509.
- ↑ a b c d e I. I. Vol'nov: Peroxides, Superoxides and Ozonides of Alkali and Alkaline Earth Metals , 1st edition, pp. 21–51, Plenum Press, New York, 1966 , no ISBN .
- ↑ a b c d e f g A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , pp. 531-538.
- ↑ Jander, Blasius, Strähle: Introduction to the inorganic-chemical practical course . 14th edition. Pp. 311-312, Hirzel Verlag, Stuttgart 1995 , ISBN 978-3-7776-0672-9 .
- ↑ EH Riesenfeld, B. Reinhold: The existence of real percarbonates and their differentiation from carbonates with crystal hydrogen peroxide , in: Reports Dtsch. Chem. Ges. 1909 , 42 , 4377-4383; doi: 10.1002 / cber.19090420428 .
- ↑ S. Gambarjan: Diphenylamine and Acylperoxides , in: Chem. Ber. , 1909 , 42 , 4003-4013; doi: 10.1002 / cber.190904203164 .
- ↑ Ullmann's Encyclopedia of Industrial Chemistry, Vol. A 19, 5th ed., Pp. 177-197, VCH, Weinheim, 1991 , ISBN 3-527-20138-6 .
- ↑ Information on environmentally friendly products for sediment oxidation and phosphate binding, company publication PDF file
- ↑ SS Medvedev, EN Alexejewa: Organic Peroxides, Part II: About the reaction between benzoyl hydroperoxide or benzoyl peroxide and triphenyl methyl , in: Chem. Ber. 1932 , 65 , 137-142; doi: 10.1002 / cber.19320650204 .
- ^ Patent du Pont de Nemours and Co. US 2357298, 1942 .
- ↑ R. Criegee , G. Müller: 1,2-Dioxane , in: Chem. Ber. 1956 , 89 , 238-240; doi: 10.1002 / cber.19560890209 .
- ^ Author collective: Organikum . 21st edition, p. 323, Wiley-VCH, 2001 , ISBN 3-527-29985-8 .
- ↑ a b c collective of authors: Organikum . 21st edition, pp. 206-207, Wiley-VCH, 2001 , ISBN 3-527-29985-8 .
- ↑ R. Brückner: reaction mechanisms . 3rd edition, pp. 41–42, Spektrum Akademischer Verlag, Munich 2004 , ISBN 3-8274-1579-9 .
- ^ AP Author (Ed.): Pathology of Oxygen , 1st Edition, pp. 25-26, Academic Press, New York 1982 , ISBN 0-12-068620-1 .
- ^ Georg Abel: Hager's handbook of pharmaceutical practice . Springer DE, 1998, ISBN 3-540-52688-9 , pp. 328 ( limited preview in Google Book search).
- ↑ Beyer-Walter: Textbook of Organic Chemistry , 23rd edition, pp. 76-77, S. Hirzel Verlag, Stuttgart · Leipzig 1998 . ISBN 3-7776-0808-4 .
- ↑ KPC Vollhardt and NE Schore: Organic Chemistry 3rd Edition, pp. 818–819, Wiley-VCH, 2000 , ISBN 3-527-29819-3 .
- ↑ J. Bülle, A. Hüttermann: The basic knowledge of organic chemistry , 1st edition, pp. 308-309, Wiley-VCH Weinheim, 2000 , ISBN 978-3-527-30847-7 .
- ↑ SS Block: Disinfection, Sterilization and Preservation , 5th ed., Lippincott, Williams & Wilki, Philadelphia, 2000 , ISBN 978-0-683-30740-5 .
- ^ WG Frankenburg: Advances in Catalysis and Related Subjects, Volume 2 , 1st Edition, pp. 24-26, 1950 , Academic Press, ISBN 978-0-12-007802-8 .
- ↑ ZW Wicks, FN Jones, SP Pappas, DA Wicks: Organic Coatings , 3rd Edition, pp. 17-26, 2007 , Wiley, New York, ISBN 978-0-470-07906-5 .
- ↑ H.–J. Arpe: Industrial Organic Chemistry: Significant preliminary and intermediate products , 6th edition, pp. 284-285, Wiley-VCH Weinheim, 2007 , ISBN 978-3-527-31540-6 .
- ↑ Thomas Brock, Michael Groteklaes, Peter Mischke - Textbook of paint technology
- ↑ Pergan: Organic peroxides for polymerisation (PDF; 2.1 MB) .
- ↑ L. Légrádi, J. Lestyán: Detection of peroxides, hydroperoxides and peracids , in: Microchimica Acta 1970 , 58 , 119-122; doi: 10.1007 / BF01218105 .
- ↑ CH Lea: The Effect of Light on the Oxidation of Fats , in: Proc. Royal Soc. London 1931 , 108 , 175-189; doi: 10.1098 / rspb.1931.0030 .
- ↑ S. Veibel: analysis of organic compounds , 1st Edition, Akademie-Verlag, Berlin, 1960 , S. 262nd
- ↑ MI Eiss, P. Gieseeke: Colorimetric Determination of Organic Peroxides , in: Anal. Chem. 1959 , 31 , 1558-1560; doi: 10.1021 / ac60153a038 .
- ↑ T. Higuchi, DA Zuek: Behaviors of Several Compounds as Indicators in Lithium Aluminum Hydride Titration of Functional Groups1 , in: J. Am. Chem. Soc. 1951 , 73 , 2676-2679; doi: 10.1021 / ja01150a073 .
- ↑ AJ Martin: Potentiometric Titration of Hydroperoxides and Peracids in Anhydrous Ethylenediamine , in: Anal. Chem. 1957 , 29 , 79-81; doi: 10.1021 / ac60121a022 .
- ^ Author collective: Organikum . 21st edition, pp. 741-762, Wiley-VCH, 2001 , ISBN 3-527-29985-8 .
- ^ DGUV regulation 13 , website of the German Social Accident Insurance, accessed on December 2, 2016.