Sharpless epoxidation
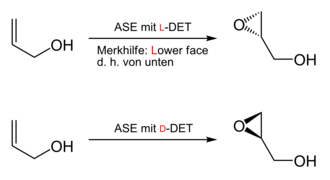
The Sharpless epoxidation is a name reaction in organic synthesis chemistry . It belongs to the group of catalytic asymmetric reactions . Starting from prochiral compounds , only one enantiomer of the product can be formed selectively ; it is an enantio- and diastereoselective reaction . The reaction yields 2,3-epoxy alcohols from allyl alcohols . These products contain both epoxy and alcohol groups and can serve as starting materials for the synthesis of numerous other substances.
Barry Sharpless received the Nobel Prize in Chemistry in 2001 for researching asymmetric oxidation reactions , which he shared with William S. Knowles and Ryoji Noyori .
Reaction conditions
Typically, the Sharpless epoxidation is carried out at −78 ° C in dichloromethane . The substrate is a primary or secondary allyl alcohol. The oxidizing agent is a hydroperoxide , mostly the tert-butyl hydroperoxide . The complex, the stereoselectivity induced formed from added in catalytic amounts of tetraisopropyl orthotitanate (titanium (IV) isopropoxide, 5 to 10 mol%) and enantiomerically pure tartaric acid diethyl ester (diethyl [DET], 6 to 12 mol%). Traces of water can react with the catalyst and deactivate it. The addition of molecular sieve (3 or 4 Å) can thus reduce the amount of catalyst used .
mechanism
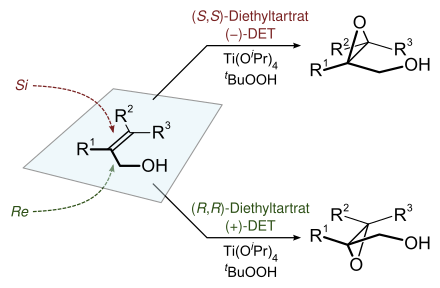
The enantioselective epoxidation proceeds from an achiral / prochiral starting material (allyl alcohol) with the aid of a chiral catalyst consisting of DET and titanium (IV) isopropoxide ( titanium tetraisopropoxide ).
To this end, titanium (IV) isopropoxide and the optically active diethyl tartrate, in the example on the right ( R, R ) or L - (+) - DET, are used to generate the chiral catalyst, which is most likely present as a dimer, with the release of two equivalents of isopropanol . The two remaining isopropanolate residues are substituted by the oxidizing agent ( tert-butyl hydroperoxide) and the allyl alcohol in the subsequent course of the reaction , creating a “complex” of chiral catalyst, allyl alcohol and epoxidation reagent. This complex has a preferred geometry (right in the picture). The geometry shown on the left is not preferred due to steric hindrance between the tert-butyl radical and the ethyl ester group . The two geometries differ for the allyl alcohol in that epoxidation would be possible from different sides. The titanium (IV) alkoxide species acts as a Lewis acid, coordinates both oxygen atoms from the peroxo group and thus activates them electrophilically, as the Lewis acid absorbs the electron density of the oxygen. The peroxo oxygen atom remote from the tert-buty radical is added to the double bond. After aqueous work-up (hydrolysis), the (2 S ) -epoxide and tert- butanol are obtained. Alternatively, the (2 R ) -epoxide is obtained by using ( S, S ) -tartaric acid diethyl ester .
Alternative reactions
Since the Sharpless epoxidation is limited exclusively to allyl alcohols, ordinary, non-functionalized olefins ( alkenes ) can alternatively be converted into epoxides ( oxiranes ) by Jacobsen-Katsuki epoxidation with optically active Mn (III) complexes or Shi epoxidation with potassium peroxomonosulfate become.
See also
literature
- Herbert Waldmann : Organic synthesis highlights II . Edition 2, Wiley-VCH, 1995, ISBN 3-527-29378-7 , pp. 3-8 ( digitized version ).
- Jie Jack Li: Sharpless asymmetric epoxidation , in: Name Reactions , Springer, Berlin / Heidelberg 2006. ISBN 978-3-540-30031-1 , 533-535, doi : 10.1007 / 3-540-30031-7_243 .
Web links
- Sharpless epoxidation at organic-chemie.ch (German)
- Sharpless epoxidation at organic-chemistry.org (English)
Individual evidence
- ↑ Tsutomu Katsuki, K. Barry Sharpless: The first practical method for asymmetric epoxidation . In: Journal of the American Chemical Society . tape 102 , no. 18 , 1980, pp. 5974-5976 , doi : 10.1021 / ja00538a077 .
- ^ Yun Gao, Janice M. Klunder, Robert M. Hanson, Hiroko Masamune, Soo Y. Ko: Catalytic asymmetric epoxidation and kinetic resolution: modified procedures including in situ derivatization . In: Journal of the American Chemical Society . tape 109 , no. 19 , September 1, 1987, pp. 5765-5780 , doi : 10.1021 / ja00253a032 .
- ^ Asymmetrical epoxidation, lecture as part of the seminar on the internship for advanced users, Leibniz University Hannover, Magnus Pfaffenbach, May 2, 2011.
- ↑ Brückner, Reinhard .: reaction mechanisms: organic reactions, stereochemistry, modern synthesis methods . 3rd edition, updated and revised. Elsevier, Spektrum, Akad. Verl, Munich 2004, ISBN 3-8274-1579-9 , pp. 139 f .