Tetraisopropyl orthotitanate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetraisopropyl orthotitanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 28 O 4 Ti | |||||||||||||||
| Brief description |
light yellow, colorless light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 284.22 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure | ||||||||||||||||
| solubility |
soluble in anhydrous ethanol , diethyl ether , benzene , chloroform , hydrolyzed in water |
|||||||||||||||
| Refractive index |
1.4640 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetraisopropyl orthotitanate or tetraisopropyl titanate , abbreviated TIPT or English TTIP (titanium tetraisopropoxide) , is formally the tetraisopropyl ester of the hypothetical orthotitanic acid (H 4 TiO 4 ). As a Lewis acid, TIPT is an important catalyst for esterification and transesterification reactions and for Sharpless epoxidations and is the starting material for ultra-thin titanium dioxide layers and nanoparticles.
Manufacturing
The equilibrium reaction of titanium tetrachloride with dry isopropanol leads to tetraisopropyl titanate Ti (OCHMe 2 ) 4 with the evolution of hydrogen chloride . To suppress the reverse reaction, the HCl gas is bound with ammonia and the ammonium chloride (NH 4 Cl) formed is filtered off.
In n- heptane as the reaction medium and by briefly heating under reflux, larger NH 4 Cl crystals form, which can be more easily filtered off. After vacuum distillation, virtually chloride-free (0.020%) TIPT is obtained in 89% yield.
properties
Pure tetraisopropyl orthotitanate is a clear, neutral (pH 7), colorless to pale yellow liquid that smells of isopropanol above room temperature . The technically pure substance contains a few percent by weight of isopropanol, which lowers the freezing point and thus makes the TIPT easier to use. In contrast to other titanic acid esters, the compound is in monomeric form in non-polar solvents. The substance is sensitive to moisture and hydrolyzes rapidly in water in an exothermic reaction to form titanium dioxide and isopropanol.
Applications
Esterification catalyst
Direct esterifications
Tetraisopropyl titanate is suitable as a neutral compound for direct esterification of carboxylic acids with alcohols with elimination of water and is used on an industrial scale in the synthesis of esters which are used as plasticizers for polyvinyl chloride (PVC).
Titanium isopropoxide reacts quickly in a transesterification reaction with free higher alcohols or glycols to form the corresponding orthotitanates - in the example with the isononanol trimethyl-3,5,5-hexanol to tetra-isononyl titanate - and isopropanol released is distilled off.
The Lewis acid formed transfers the isononanol residue with ester formation to the carboxy group of the monoester to form 1,2-cyclohexanedicarboxylic acid diisononyl ester (DINCH) and reacts with free alcohol back to the tetraisononyl titanate.
In contrast to acidic and basic esterification catalysts, significantly fewer colored by-products are formed under titanate catalysis, which is why larger excesses of alcohol (up to 25%) can be used to shift the equilibrium. High conversion and product purity make the neutralization and washing steps usually necessary for esterifications superfluous. Tetraisopropyl titanate concentrations of only 0.05 to 0.2%, based on the ester product, are usually required.
Transesterifications
In the case of transesterifications with tetraisopropyl titanate, the tetraalkyl orthotitanate initially formed as in esterifications catalyzes the exchange of the alkoxy group of the starting ester with that of the alcohol
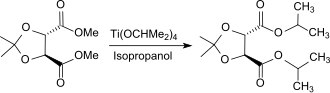
Because of the low tendency to side reactions when using titanate transesterification catalysts, easily polymerizable esters such. B. methyl acrylate H 2 C = CH-COOCH 3 , with functional alcohols, such as. B. amino alcohols such as dimethylaminoethanol HO- (CH 2 ) 2 -N (CH 3 ) 2 can be produced with yields of up to 98%. Even esters with acid-sensitive groups, such as. B. acetals or ketals (example: dimethyl-2,3- O -isopropylidene tartrate) can be transesterified smoothly with TIPT as a catalyst.
The yield is 91 to 95%. In this way, transesterifications in the presence of alkyne and nitrile groups , β-lactams, trimethylsilyl groups , and Boc and other carbamate protective groups can also be carried out gently and efficiently.
Polycondensation
Titanium isopropoxide as a component of catalyst systems together with antimony trioxide also played a role in the transesterification of dimethyl terephthalate with ethylene glycol to polyethylene terephthalate PET, which is largely replaced today by the process of direct esterification of terephthalic acid with ethylene glycol .
In the direct esterification of terephthalic acid with ethylene glycol and subsequent polycondensation, when using tetraisopropyl titanate, the amount of the commonly used antimony trioxide can be reduced and even eliminated, and PET with mechanical and optical properties suitable for fibers, films and beverage bottles can be obtained.
Catalyst for the Ziegler-Natta polymerization
The so-called Phillips catalysts of the second generation for the polymerization of ethene and propene consist of magnesium - titanium compounds and triethylaluminum as a cocatalyst. The titanium component is often introduced via titanium isopropoxide. The catalyst systems are significantly (10 to 100 times) more active than the first generation catalysts and can therefore remain in the product. They enable the production of linear low-density polyethylene ( linear low-density polyethylene , LLDPE) and high-density polyethylene (HDPE) with a narrow molecular weight distribution .
Sharpless epoxidation catalyst
In the presence of tetraisopropyl titanate and (+) - or (-) - diethyl tartrate in dichloromethane at −70 to −20 ° C, achiral primary allyl alcohols are enantioselectively epoxidized. A classic example is the epoxidation of geraniol with tert-butyl hydroperoxide to geraniol-2,3-oxide in very high chemical yield and high enantiomeric excess
The pronounced tendency of orthotitanates to react with the active hydrogen atoms of hydroxyl, amino, amido, carboxyl and thiol groups with crosslinking and formation of thin amorphous TiO 2 layers can be used to modify glass, metal and polymer surfaces in a variety of ways such as B. as an adhesion promoter ( primer ) to increase the adhesion , the hardness and the abrasion and scratch resistance, the thermal and chemical resistance, the light reflection and the corrosion resistance.
Such coatings also increase the dispersibility of pigments and fillers in water-based or non-aqueous lacquers and paints and reduce their viscosity . On the other hand, by crosslinking hydroxyl-containing polymers in paints with titanium isopropoxide, the viscosity can also be increased, the thixotropy of latex paints adjusted and their adhesion to surfaces improved. The crosslinking properties of tetraisopropyl titanate make it an effective additive in papermaking to increase wet strength, in oil drilling aids to control viscosity and in printing inks to improve adhesion.
Titanium dioxide particles and films
Using carefully controlled hydrolytic sol-gel processes or pyrolytic processes at temperatures above 350 ° C, not only thin polymeric TiO 2 films but also micro- and nano-scale TiO 2 particles can be produced from titanium isopropoxide . Because of their high refractive index and UV absorption, these particles have interesting potentials as photocatalysts , in photovoltaics and for applications in lighting and signage.
Metal titanates
Tetraisopropyl titanate forms with carboxylic acids , such as. B. acetic acid non-stoichiometric oligomeric titanium acylates of the approximate composition Ti (OOCMe) 2 (OCHMe 2 ) 2 , which react with alkaline earth metal carbonates to form the corresponding alkaline earth metal titanates, which are of interest for the construction of multilayer ceramic capacitors and thermistors because of their ferroelectric properties .
Manufacturer
Former manufacturers DuPont and Johnson Matthey sold their businesses and trademarks for orthotitanates in 2010 [Tyzor TPT (DuPont) and VERTEC TIPT (Johnson Matthey) for tetraisopropyl titanate] to Dorf Ketal Chemicals (India) Pvt. Ltd. After the production of Johnson Matthey in Billingham , UK was discontinued in 2011, tetraisopropyl titanate will only be produced on a large scale in India (Ketal village) and China (Borica Co., Ltd. from Taiwan under the trademark Tytan TM TIPT).
literature
- DE Putzig, JR Moncarz: Titanium Compounds, Organic in Kirk-Othmer Encyclopedia of Chemical Technology . 2005, doi : 10.1002 / 0471238961.1518070116212026.a01.pub2 .
Individual evidence
- ↑ a b c d e f g Entry on titanium tetraisopropanolate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c data sheet Titanium (IV) isopropoxide from Sigma-Aldrich , accessed on February 20, 2015 ( PDF ).
- ↑ a b c d data sheet Titanium (IV) isopropoxide at AlfaAesar, accessed on February 20, 2015 ( PDF )(JavaScript required) .
- ↑ a b Patent US3119852 : Preparation of organic titanates. Applied on August 11, 1960 , published January 28, 1964 , applicant: EI du Pont de Nemours & Co., inventor: RT Gilsdorf.
- ↑ a b René Imwinkelried, Martin Schiess, Dieter Seebach: Diisopropyl (2S, 3S) -2,3-O-isopropylidenetartrate In: Organic Syntheses . 65, 1987, p. 230, doi : 10.15227 / orgsyn.065.0230 ; Coll. Vol. 8, 1993, p. 201 ( PDF ).
- ↑ a b Titanium (IV) isopropoxide data sheet (PDF) from Fisher Scientific , accessed on May 25, 2017.
- ↑ Patent US2187821 : Preparation of titanium alcoholates and phenolates. Registered on March 2, 1937 , published on January 23, 1940 , applicant: IG Farbenindustrie AG, inventor: J. Nelles.
- ↑ Patent WO2006136471A1 : Mixture of diisononyl esters of 1,2-cyclohexanedicarboxylic acid, process for their preparation and use of these mixtures. Applied on April 27, 2006 , published on December 28, 2006 , applicant: Oxeno Olefinchemie GmbH, inventor: M. Grass, A. Lang.
- ↑ Johnson Matthey brochure: Johnson Matthey Catalysts: VERTEC ® - Direct Esterification Technology , PDF ( Memento of the original from March 6, 2007 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent DE10127939A1 : Process for the production of (meth) acrylic acid esters. Registered on June 8, 2001 , published on May 29, 2002 , applicant: BASF AG, inventor: M. Geissendörfer, A. Dams, G. Nestler.
- ↑ Patent US4356299 : Catalyst system for a polyethylene terephthalate polycondensation. Filed February 4, 1982 , published October 26, 1982 , Applicant: Rohm and Haas Co., Inventor: MS Cholod, NM Shah.
- ↑ Johnson Matthey Catalysts, VERTEC Polyester Catalyst technology for PET fiber , 2003, PDF ( Memento of the original from March 6, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent US4452914 : Titanium complexes and catalysts prepared therefrom. Filed August 13, 1982 , published June 4, 1984 , Applicant: The Dow Chemical Co., Inventor: WM Coleman III, MS Edmonson.
- ↑ TE Nowlin, RI Mink, YV Kissin: Supported Magnesium / Titanium-Based Ziegler Catalysts for Production of Polyethylene . Ed .: R. Hoff, RT Mathers. Wiley & Sons, Inc., 2010, ISBN 978-0-470-13798-7 , pp. 131-156 .
- ^ DB Malpass: Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes . Scrivener Publishing LLC, 2010, ISBN 978-0-470-62598-9 .
- ↑ T. Katsuki, KB Sharpless: The first practical method for asymmetric epoxidation . In: J. Am. Chem. Soc. tape 102 , no. 18 , 1980, pp. 5974-5976 , doi : 10.1021 / ja00538a077 .
- ↑ J. Gordon Hill, K. Barry Sharpless, Christopher M. Exon, and Ronald Regenye: Enantioselective epoxidation of allylic alcohols: (2S, 3S) -3-propyloxiranemethanol In: Organic Syntheses . 63, 1985, p. 66, doi : 10.15227 / orgsyn.063.0066 ; Coll. Vol. 7, 1990, p. 461 ( PDF ).
- ↑ DuPontTM TyzorR Organic Titanates: General Brochure , PDF ( Memento of the original from June 29, 2015 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent US7943116 : High-yield synthesis of brookite TiO 2 nanoparticles. Filed January 24, 2008 , published May 17, 2011 , applicant: Sandia Corp., inventor: DL Huber, TC Monson.
- ↑ DP Birnie III, NJ Bendzkob: 1 H and 13 C NMR observation of the reaction of acetic acid with titanium isopropoxide . In: Mater. Chem. Phys. tape 59 , no. 1 , 1999, p. 26-35 , doi : 10.1016 / S0254-0584 (99) 00021-8 .
- ↑ Patent US4670243 : Method of precipitating metal titanate powders. Filed June 30, 1986 , published June 2, 1987 , Applicant: Ferro Corp., Inventor: JM Wilson, DL Coller, S. Venkataramani.
- ↑ VILLAGE KETAL ACQUIRES DUPONT CHEMICALS AND FLUOROPRODUCTS SPECIALITY CATALYSTS BUSINESS , PDF
- ↑ Johnson Matthey Announces Divestment of its Vertec Business , online ( Memento of the original dated February 27, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.


![{\ displaystyle \ mathrm {TiCl_ {4} +4 (CH_ {3}) _ {2} CHOH {\ xrightarrow [{- NH_ {4} Cl}] {+ NH_ {3}}} Ti [OCH (CH_ { 3}) _ {2}] _ {4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e259838b6f019dee284d532a502a0aa30d4d6e9e)