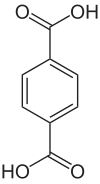
Terephthalic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Terephthalic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 6 O 4 | |||||||||||||||
| Brief description |
colorless, crystalline solid with a sour odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 166.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.51 g cm −3 (20 ° C) |
|||||||||||||||
| Sublimation point |
402 ° C |
|||||||||||||||
| Vapor pressure |
1.33 h Pa (78 ° C) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
practically insoluble in water : 15 mg · l −1 (20 ° C), alcohols and ethers, more soluble in hot sulfuric acid , pyridine , dimethyl sulfoxide and dimethylformamide |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
5 mg m −3 (measured as the inhalable aerosol fraction ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Terephthalic acid or para- phthalic acid is an organic chemical compound and belongs to the aromatic dicarboxylic acids ( benzene dicarboxylic acids ). The name of the acid is derived from oil of turpentine , through the oxidation of which it was first made, and from phthalic acid , to which it is isomeric . It is usually used in the form of a colorless, free-flowing powder for the production of saturated polyesters . Constitutional isomers are phthalic acid and isophthalic acid . Due to the rapidly growing production volumes of the polyester polyethylene terephthalate (PET), the consumption of the main monomer terephthalic acid is estimated at around 50 million tons in 2012. This makes terephthalic acid one of the world's largest organic chemical products in terms of volume.
Extraction and presentation
Synthesis from fossil raw materials
Terephthalic acid can be produced from p -xylene by air oxidation with the aid of cobalt naphthalate as catalyst manufacture. This synthesis route was first discovered in 1951 by Ewald Katzschmann .
Most of these catalysts today are combinations of cobalt , manganese and bromine or cobalt with a co-oxidant, for example acetaldehyde . The reaction solvent used is 95% acetic acid and a catalyst combination of cobalt and manganese acetate (Amoco process). The added bromine compound (NH 4 Br, tetrabromomethane , Co - and manganese (II) bromide or HBr ) functions as a promoter and radical carrier. The oxidation with atmospheric oxygen takes place in modern process variants in bubble column reactors with> 150 m³ capacity in the liquid phase at elevated temperature (> 150 ° C, mostly 180–210 ° C) and pressure (1,500–3,000 kPa) because of the corrosiveness of the reaction mixture Titan or Hastelloy C reactors instead. The enthalpy of reaction is removed by evaporative cooling of the evaporating acetic acid. The reaction proceeds with an approximately 96% yield of terephthalic acid and, via hydroperoxides formed as intermediates, initially leads to p -tolualdehyde and p -toluic acid . Their methyl group is strongly deactivated due to the electron-withdrawing influence of the para carboxy group , so that further oxidation is much more complex. Another complication is the formation of 4-carboxybenzaldehyde (4-CBA or 4-formylbenzoic acid), which turns the end product yellow. 4-CBA is converted into p -toluic acid by catalytic hydrogenation by catalytic hydrogenation after the reaction mixture, which is obtained as a suspension, has been discharged from the reactor, the hydrous acetic acid has been removed and the crude terephthalic acid has been dissolved in water and then returned to the oxidation reaction.
The resulting “fiber-pure” terephthalic acid ( English purified terephthalic acid , PTA) contains <25 ppm 4-CBA and has a purity of> 99.99%, as it is necessary to achieve high molar masses in the polycondensation with diols to form polyesters . With a gas mixture of oxygen and carbon dioxide , the oxidation reaction proceeds significantly faster (+26%) and more selectively, since the formation of 4-CBA is suppressed, which should lead to considerable cost savings.
With the technical maturity of the air oxidation process for high-purity terephthalic acid, other oxidation processes with nitric acid or process variants that lead to dimethyl terephthalate (DMT) became obsolete.
The isomerization of dipotassium phthalate to dipotassium terephthalate (so-called Henkel-I process), as well as the disproportionation of potassium benzoate to dipotassium terephthalate and benzene (Henkel-II process) (Henkel-II process) are also out of date , since the complete recycling of the potassium could not be solved economically. Even procedures that an ammoxidation of p -xylene to terephthalonitrile run and subsequent hydrolysis have not been successful because of the complex nitrogenous contaminants to be removed in practice.
According to modern variants of the Amoco process , plants for the production of PTA with annual capacities of over 1 million tons / year and individual reactors with annual capacities of over 100,000 tons / year are currently being built. The process is also suitable for the direct oxidation of other methylated benzenes or naphthalenes to the corresponding aromatic carboxylic acids , such as. B. 2,6-naphthalenedicarboxylic acid from 2,6-dimethylnaphthalene .
Other processes are based on the carboxylation of benzoic acid with potassium hydrogen carbonate ( Kolbe-Schmitt reaction ) and the isomerization of potassium phthalate .
Synthesis from renewable raw materials
The initiative of major soft drink manufacturers to introduce beverage bottles made of “bio-PET”, which is to be made from monomers derived entirely from renewable raw materials, is moving alternative routes to terephthalic acid, based on (potentially) large-volume biomolecules , into the focus of extensive research activities.
The following synthesis routes are considered to be promising, in which the C 8 aromatic p -xylene is obtained by linking biogenic C 2n reactants (e.g. C 6 plus C 2 in process 1.) or by oxidative removal of surplus substituents (C 10 minus C 2 in process 3.) and subsequent aromatization is generated:
1. Diels-Alder reaction (addition) of ethylene on 2,5-dimethylfuran to p -xylene in a C 6 + C 2 linkage (process of UOP LLC):
2,5-Dimethylfuran can be obtained in good yields from hexoses such as glucose or fructose via hydroxymethylfurfural and recently also directly from cellulose . With ethylene from bioethanol , all of the raw materials come from renewable sources.
2. Dehydration of isobutanol to isobutene , dimerization to isooctene and cyclization to p -xylene:
Starting from isobutanol, which is now available in larger quantities through fermentation of sugars or cellulose, a number of C 8 compounds are generated in a C 4 + C 4 linkage in a multi-stage reaction sequence , which are also of interest as alternative fuels. The disadvantage of a longer synthetic route is offset by the advantage of using refinery processes implemented on a large scale (isobutene oligomerization) (processes from Gevo Inc. and Butamax Advanced Biofuels LLC ).
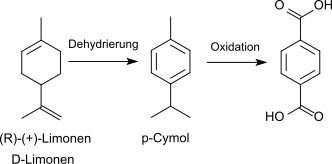
3. Dehydration and subsequent oxidation of D- limonene to terephthalic acid:
The process starts from cheap ( R ) - (+) - limonene from orange peel , but requires oxidizing agents such as iron (III) chloride , nitric acid and potassium permanganate , which are unsuitable for transfer to an industrial scale, to achieve high yields (process the SABIC).
4. Zeolite-catalyzed conversion of biomass to BTX aromatics. Here, in a single-stage “catalytic pyrolysis”, shredded biomass such as wood waste, plant residues etc. is converted into a BTX mixture at approx. 600 ° C in a fluidized bed reactor on a modified zeolite contact with a yield of 40%. The BTX mixture is worked up by distillation on p-xylene (method of Anellotech, Inc.). Based on preliminary cost calculations, this route appears to be able to compete with the production costs of petroleum-based PTA.
5. Conversion of trans , trans - muconic acid (2,4-hexadiene diacid) from glucose to terephthalic acid:
Muconic acid reacts here as a dienophile with ethylene in a Diels-Alder reaction in a C 6 + C 2 linkage without the detour via p -xylene directly to the terephthalic acid precursor 1,4-cyclohexenedicarboxylic acid, which is dehydrogenated to terephthalic acid (Amyris process , Inc.).
6. Conversion of soluble carbohydrates into p -xylene. The process (BioForming® process) converts pentoses (C 5 sugar) and hexoses (C 6 sugar) by means of a so-called aqueous phase reforming (APR) step at elevated temperature (170-300 ° C) and pressure (10-90 bar) sugar to a mixture of low-oxygen acyclic and cyclic molecules, from which BTX aromatics are obtained through acid-catalyzed condensation, from which in turn p- xylene is conventionally separated and this is further converted into PTA (process of Virent Energy Systems, Inc.). At first glance, processes based on the principle of “biomass to biomonomers” appear to be the most promising (see process 4.), but due to the low energy density of biomass and the associated high transport costs, only decentralized systems with capacities of up to 250,000 tons per year are realistic. In addition, from the point of view of the synthetic chemist, it does not seem elegant to reduce oxygen-rich biomolecules with a "fire and sword chemistry" to the hydrocarbon p-xylene in order to then oxidize it to terephthalic acid in a laborious process.
None of the new processes has yet been implemented in large-scale practice, as parameters such as raw material prices and availability, CAPEX (investment costs ) and OPEX (operating costs, influenced by stable high space-time yields , catalyst service lives , energy balances, etc.) play a decisive role . It remains to be seen whether, in future, bio-terephthalic acid plants with conventional PTA plants will be able to keep up with today's standard capacity of 1.4 to 2.2 million tons per year.
properties
Terephthalic acid can form needle-shaped crystals through slow crystallization .
It is sparingly soluble in most solvents. The best solubilities (> 10 g in 100 g solvent at normal temperature) are achieved with ammonia solution , sodium hydroxide solution , dimethylformamide and dimethyl sulfoxide .
| Solubilities at 25 ° C | |
|---|---|
| water | 0.0017 g / 100 g |
| Glacial acetic acid | 0.013 g / 100 g |
| Methanol | 0.1 g / 100 g |
| Dimethylformamide | 6.7 g / 100 g |
| Dimethyl sulfoxide | 19.0 g / 100 g |
Due to the low solubility of terephthalic acid in water and alcohols, the technical acid obtained from p-xylene in earlier oxidation processes was purified to fiber-pure DMT via the diester dimethyl terephthalate (DMT) by vacuum distillation and recrystallization from methanol or xylene and then hydrolyzed again to terephthalic acid.
use
It is mainly used for the production of saturated polyesters with aliphatic diols as comonomers . Around 90 percent of the annual production of terephthalic acid goes into the production of the plastic polyethylene terephthalate (PET) (approx. 2/3 of the total amount) and food packaging (approx. 1/3), in particular beverage bottles. Ethylene glycol and up to 5 mol% isophthalic acid and diethylene glycol are used as comonomers . The annual production volume of terephthalic acid in 2006 was 37.3 million tons, by 2014 the PTA capacity is expected to increase by more than 15 million tons, of which approx. 68% in China. Several hundred thousand tons of terephthalic acid are used in the production of technical polyesters such as polybutylene terephthalate (PBT) for thermally demanding automotive applications in the engine compartment and relatively small amounts in aromatic polyamides of the aramid type (e.g. Kevlar ™ ) for highly tear-resistant fibers.
safety instructions
Terephthalic acid is not very toxic. Contact can cause slight, reversible irritation of the skin, eyes and respiratory tract. The oral LD 50 is 18.8 g / kg body weight for rats and 6.4 g / kg for mice. When rats were ingested high doses (around 3 percent) mixed with the feed, calcium terephthalate bladder stones formed in rats . These stones injured the bladder and lead to cancer.
Terephthalic acid dust can form explosive mixtures with the air. The minimum concentration of terephthalic acid required for an explosion is 40 g · m −3 at 20 ° C, the required oxygen content is 12.4 percent. At 150 ° C, only 11.1 percent of oxygen is required. It was calculated that above a concentration of 1400 g · m −3 terephthalic acid no more explosion occurs.
The flash point of terephthalic acid is 260 ° C and the ignition temperature is 678 ° C. The reaction with strong oxidizing agents can generate heat.
Web links
literature
- Barbara Elvens u. a. (Ed.): Ullmann's Encyclopedia of Chemical Industry . 5th edition VCH, Weinheim 1985, ISBN 3-527-20100-9 (former title: Ullmanns Enzyklopädie der technischen Chemie ).
- Jacqueline I. Kroschwitz (Ed.): Encyclopedia of Chemical Technology . 4th ed. Wiley, New York 2001, ISBN 0-471-41961-3 .
- Entry to terephthalic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
Individual evidence
- ↑ a b c d e f g Entry on terephthalic acid in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ OECD: SIDS , accessed January 8, 2019
- ↑ a b CRC Handbook of Tables for Organic Compound Identification . Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 100-21-0 or terephthalic acid ), accessed on November 2, 2015.
- ↑ a b Beyer-Walter textbook on organic chemistry . S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 .
- ↑ Ph. Gibbs, An Assessment of The Key Drivers of Global Polyester and Raw Material Markets ( Memento from January 20, 2013 in the Internet Archive ) (PDF file; 2.1 MB), IOCL Petrochem Conclave 2012, PCI Xylenes & Polyesters Ltd , March 2012.
- ↑ Patent application DE000C0010059 : Process for the production of terephthalic acid. Registered on October 6, 1954 , published on September 9, 2005 , applicant: Chemische Werke Hüls, inventors: Franz Broich, Gunthard Hoffmann, Ferdinand List.
- ↑ a b c H.-J. Arpe, Industrial Organic Chemistry. 6. Completely revised. Ed., Wiley-VCH, Weinheim, January 2007, ISBN 978-3-527-31540-6 .
- ↑ NN, Terephthalic acid methods ( Memento from June 13, 2013 in the Internet Archive ) (PDF file; 46 kB).
- ↑ Y. Xiao et al., Aerobic Oxidation of p-Toluic Acid to Terephthalic Acid over T (p-Cl) PPMnCl / Co (OAc) 2 Under Moderate Conditions , Catalysis Letters, 134, pp. 155–161 (2010), doi: 10.1007 / s10562-009-0227-1 .
- ↑ US Patent US 6,194,607, Methods of producing aromatic carboxylic acids by oxidizing alkyl aromatic hydrocarbons or partially oxidized intermediates thereof , inventor: S.-H. Jhung et al., Applicant: Samsung General Chemicals Co., Ltd., issued February 27, 2001.
- ↑ US Patent US 5,183,933, Process for preparaing 2,6-naphthalene-dicarboxylic acid , inventor: JJ Harper et al., Applicant: Amoco Corp., issued February 2, 1993.
- ↑ ICIS News : US Coca-Cola partners with Gevo, Virent, Avantium on bio-PET , published December 15, 2011.
- ↑ NNFCC members-only Update: April 2011 Renewable Chemicals and Materials .
- ^ ICIS Chemical Business : Development of bio-paraxylene and PTA on the rise , March 12, 2012.
- ↑ US Patent Application US 2010/0331568, Carbohydrate route to para-xylene and terephthalic acid , inventor: TA Brandvold, applicant: Honeywell / UOP Patent Services, published December 30, 2010.
- ↑ Y. Román-Leshkov et al. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates , Nature 447, 982-985 (21 June 2007), doi: 10.1038 / nature05923 .
- ↑ BJ Caes et al., Organocatalytic conversion of cellulose into a platform chemical , In: Chem Sci ., 2013, 4, 196, doi: 10.1039 / c2sc21403b .
- ↑ US patent application US 2011/0087000, Integrated Process to Selectively Convert Renewable Isobutanol to P-Xylene , inventor: MW Peters et al., Applicant: GEVO, Inc., published April 14, 2011.
- ↑ US patent US 8,283,505, Recovery of higher alcohols from dilute aqueous solutions , inventor: WA Evanko et al., Applicant: Gevo, Inc., issued October 9, 2012.
- ↑ US patent US 8,178,328, Fermentative production of four carbon alcohols , inventor: GK Donaldson et al., Applicant: Butamax Advanced Biofuels LLC, issued May 15, 2012.
- ^ DA Glassner: Hydrocarbon Fuels from Plant Biomass ( Memento from April 21, 2013 in the Internet Archive ). 2009, Gevo, Inc. (PDF; 837 kB).
- ↑ Gevo Overview Berlin Air Show ( Memento of the original from December 5, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF file; 2.1 MB), September 2012.
- ↑ Gevo White Paper, Transportation Fuels ( Memento of the original from April 29, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF file; 2.2 MB), May 2011.
- ↑ WIPO application WO 2010/078328 A2, Bio-based terephthalate polyesters , inventor: C. Berti et al., Applicant: SABIC Innovative Plastics IP BV, published on July 8, 2010.
- ↑ Y.-T. Cheng et al., Production of Renewable Aromatic Compounds by Catalytic Fast Pyrolysis of Lignocellulosic Biomass with Bifunctional Ga / ZSM-5 Catalysts , Angew. Chem., 124 , (6), pp. 1416-1419 [2012], doi: 10.1002 / anie.201107390 .
- ↑ K. Bourzac, From biomass to chemicals in one step , MIT Technology Review , March 29, 2010.
- ^ ICIS Chemical Business, Development of bio-paraxylene and PTA on the rise , published March 12, 2012.
- ↑ US patent application US 2011/0124911, Semi-synthetic terephthalic acid via microorganisms that produce muconic acid , inventor: MJ Burk et al., Published on May 26, 2011.
- ↑ PCT patent application WO 2010/148049, Preparation of trans, trans muconic acid and trans, trans muconates , inventor: JW Frost et al., Applicant: Draths Corp., published on December 23, 2010.
- ↑ G. Keenan, 100% Biobased PET: A Sustainable Approach to Fiber, Film, and Bottles ( Memento of the original from December 4, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF file; 1.3 MB), Virent Inc., 2012.
- ↑ D. Komula, Completing the Puzzle: 100% Plant-Derived PET (PDF file; 2.1 MB), bioplastics MAGAZINE [04/11] Vol. 6.
- ^ ICIS Chemical Business, PTA expansions threaten margins , published May 28, 2012.
- ↑ CEH Marketing Research Report, Dimethylterephthalate (DMT) and Terephthalic Acid (TPA) , May 2007.
- ^ IHS Chemical Report, Dimethyl Terephthalate (DMT) and Terephthalic Acid (TPA) , published August 2010.