Substitution pattern
The substitution patterns are part of the chemical nomenclature and describe the relative positions of the substituents in aromatic carbon skeletons.
ortho , meta , para
The Greek prefixes ortho (from ὀρθός = "upright", "straight"), meta (from μετά = "after", "beyond") and para (from παρά = "next to", "in spite of", "against [above]" ) in organic chemistry denote the position of a second substituent in relation to the first substituent, usually on the benzene ring .
 |
 |
 |
|
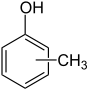
| o - cresol | m - cresol | p - cresol | Cresols * |
The terms are placed as a descriptor in front of the molecule name. They are often also abbreviated as o - (for ortho), m - (for meta) and p - (for para). Ortho denotes a 1,2, meta a 1,3 and para a 1,4 substitution. There are no other isomers : the product of a 1,5 substitution is the mirror image of the product of a 1,3 substitution and is identical to it. The terms do not correspond to the nomenclature recommendations of the IUPAC , but have been preserved in the form of numerous common names in the language of chemists to this day. For example, the common name p-xylene is far more common than the correct name 1,4-dimethylbenzene.
vicinal (vic.), geminal ( mixed ), asymmetrical (asym.), symmetrical (sym.)
In chemistry , vicinal (lat. Vicinus = neighbor) means that two functional groups are bound to two adjacent carbon atoms. This designation is also transferred to the 1,2,3-trisubstituted aromatics (e.g. pyrogallol ). When two functional groups are attached to the same carbon atom this is called a geminal substitution pattern.
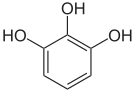
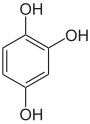

Pyrogallol ,
1,2,3-trihydroxybenzene,
vic. -TrihydroxybenzeneHydroxy hydroquinone ,
1,2,4-trihydroxybenzene,
asym. -TrihydroxybenzolPhloroglucinol ,
1,3,5-trihydroxybenzene,
sym. -Trihydroxybenzol
ipso , meso , peri
- The ipso substitution (Latin ipso = self) expresses that two substituents take the same place in the ring, e.g. B. as a transition state in an electrophilic aromatic substitution .
- The meso substitution (Gr. Μέσος = in the middle , middle) refers to substituents that are in the benzylic position.
- The peri-substitution (Gr. Περί = around - around; against) is a special case in naphthalenes for substituents in the 1- and 8-position.
- The prefix meso- is also used to denote symmetrical molecular forms with several (even-numbered), mutually compensating, stereogenic centers, e.g. B. meso - tartaric acid used.
cine and tele
- The cine substitution (Greek κινέ = to move) describes the new position of the entry group on the atom next to the position of the leaving group.
- The tele-substitution (Gr. Τῆλε = far) occurs when the new position of the entering group is more than one atom away from the position of the leaving group.
Individual evidence
- ^ Wolfgang Holland: The nomenclature in organic chemistry , VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1969, page 38.
- ^ Brockhaus ABC Chemie in two volumes, VEB FA Brockhaus Verlag Leipzig, 1965, p. 861.
- ^ Entry on cine-substitution . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01081 Version: 2.3.1.
- ↑ Entry on tele-substitution . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.T06256 Version: 2.3.1.



